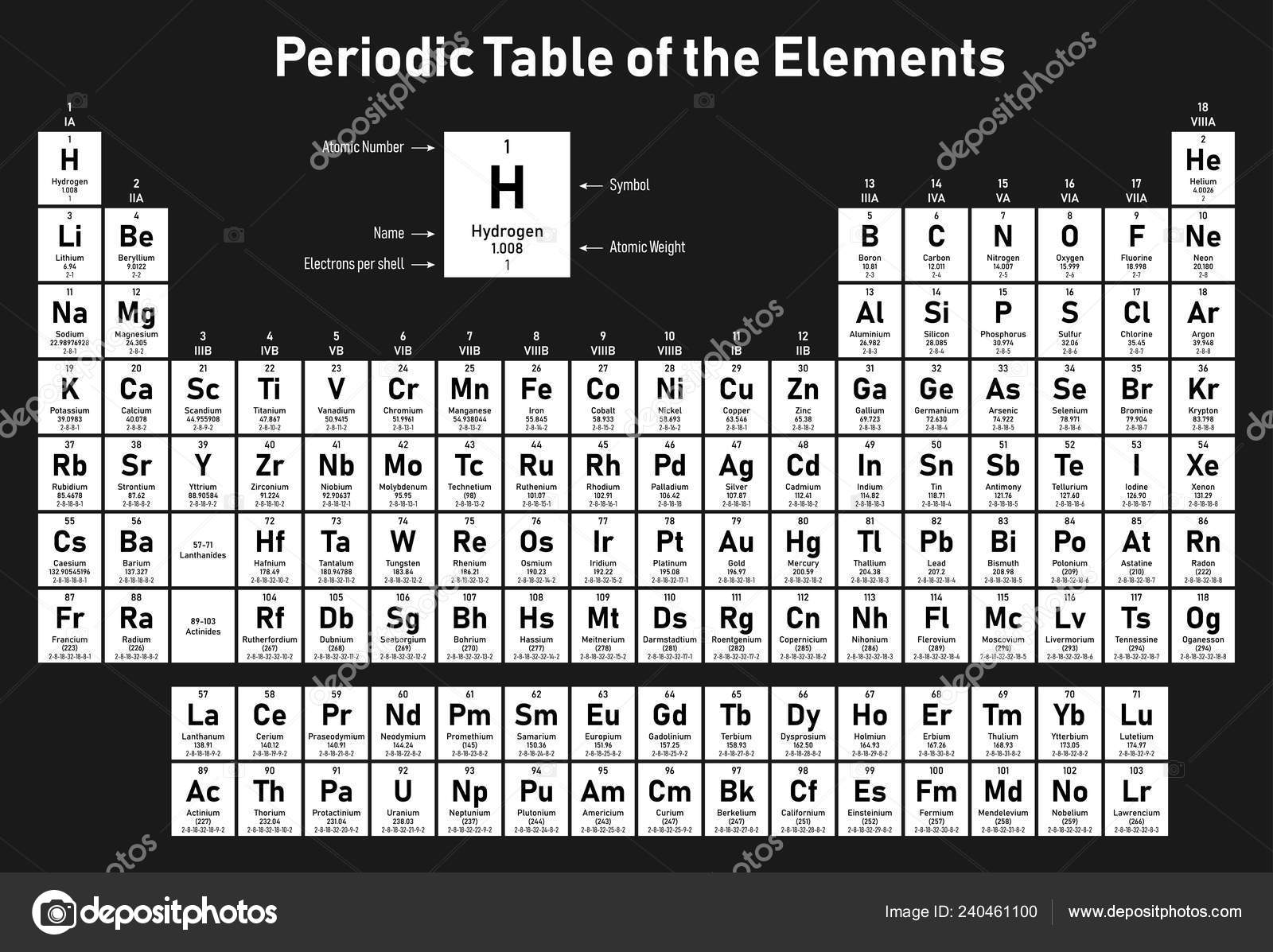
Element X Y Z Have Atomic Number 19 37 55 . three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. Which of the following statements is false about. Within a group, ie decreases from top to bottom. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. As atomic number, increases, the ionization. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals).
from depositphotos.com
elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. As atomic number, increases, the ionization. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Which of the following statements is false about. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). Within a group, ie decreases from top to bottom.
Periodic Table Elements Shows Atomic Number Symbol Name Atomic Weight
Element X Y Z Have Atomic Number 19 37 55 elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). Which of the following statements is false about. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. Within a group, ie decreases from top to bottom. As atomic number, increases, the ionization. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). As atomic number, increases, the ionization. three elements x , y and z have atomic. Element X Y Z Have Atomic Number 19 37 55.
From anatomyandphysiologyi.com
Atoms and Elements Anatomy & Physiology Element X Y Z Have Atomic Number 19 37 55 (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. Which of the following statements is false about. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals).. Element X Y Z Have Atomic Number 19 37 55.
From infinitylearn.com
Modern Periodic Table, Law, Group, Elements and Atomic Number Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. Which of the following statements is false about. The elements x (19), y (37) and z (55) are the elements of same group (ia). elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic. Element X Y Z Have Atomic Number 19 37 55.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Element X Y Z Have Atomic Number 19 37 55 (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. As atomic number, increases, the ionization. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with. Element X Y Z Have Atomic Number 19 37 55.
From thechemistrynotes.com
Periodic Table of Elements Definition, Terms, 118 Elements Element X Y Z Have Atomic Number 19 37 55 As atomic number, increases, the ionization. Within a group, ie decreases from top to bottom. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. three elements x , y and z have atomic numbers 19 ,. Element X Y Z Have Atomic Number 19 37 55.
From awesomehome.co
Periodic Table Of Elements List With Protons Neutrons And Electrons Element X Y Z Have Atomic Number 19 37 55 elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). Within a group, ie decreases from top to bottom. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential. Element X Y Z Have Atomic Number 19 37 55.
From mungfali.com
Periodic Table With Names And Atomic Mass Element X Y Z Have Atomic Number 19 37 55 (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. elements x, y and z with atomic numbers. Element X Y Z Have Atomic Number 19 37 55.
From www.crushpixel.com
Helles buntes Periodensystem der Elemente mit Atommasse Element X Y Z Have Atomic Number 19 37 55 elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). Which of the following statements is false about. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). . Element X Y Z Have Atomic Number 19 37 55.
From www.vectorstock.com
Periodic table of the elements with atomic number Vector Image Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. As atomic number, increases, the ionization. elements x, y and. Element X Y Z Have Atomic Number 19 37 55.
From courses.lumenlearning.com
Reading The Periodic Table of Elements Biology (Early Release) Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. As atomic number, increases, the ionization. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). The elements x (19), y (37) and z. Element X Y Z Have Atomic Number 19 37 55.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Element X Y Z Have Atomic Number 19 37 55 The elements x (19), y (37) and z (55) are the elements of same group (ia). Which of the following statements is false about. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. As atomic number, increases, the ionization. (a) their ionization potential would increases with increasing atomic number ( b. Element X Y Z Have Atomic Number 19 37 55.
From chemassist.blogspot.com
ChemAssist Elements and the Periodic Table Element X Y Z Have Atomic Number 19 37 55 elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). As atomic number, increases, the ionization. The elements x (19), y (37) and z (55) are the elements of same group (ia). (a) their ionization potential would increases. Element X Y Z Have Atomic Number 19 37 55.
From elchoroukhost.net
Periodic Table Of The Elements Definition Biology Elcho Table Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. elements ′x′,′y ′ and ′z′ have atomic numbers. Element X Y Z Have Atomic Number 19 37 55.
From www.youtube.com
Three elements X,Y, and Z have atomic numbers 19, 37, and 55 Element X Y Z Have Atomic Number 19 37 55 elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. As atomic number, increases, the ionization. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Within a group, ie decreases from top to bottom. (a) their ionization potential would increases with increasing atomic number ( b ) 'y'. Element X Y Z Have Atomic Number 19 37 55.
From www.vrogue.co
Chemical Elements Chart 1 Printable Atomic Number Nam vrogue.co Element X Y Z Have Atomic Number 19 37 55 Within a group, ie decreases from top to bottom. The elements x (19), y (37) and z (55) are the elements of same group (ia). (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. As. Element X Y Z Have Atomic Number 19 37 55.
From stock.adobe.com
Periodic table of elements, with element name, element symbols, atomic Element X Y Z Have Atomic Number 19 37 55 Within a group, ie decreases from top to bottom. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Which of the following statements is false about. (a) their ionization potential would increases with. Element X Y Z Have Atomic Number 19 37 55.
From www.britannica.com
Elements of the Periodic Table Quiz Britannica Element X Y Z Have Atomic Number 19 37 55 Which of the following statements is false about. As atomic number, increases, the ionization. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. three elements x , y and z have atomic numbers 19. Element X Y Z Have Atomic Number 19 37 55.
From printable.conaresvirtual.edu.sv
Periodic Table With Names Printable Element X Y Z Have Atomic Number 19 37 55 elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). Which of the following statements is false about. The elements x (19), y (37) and z (55) are the elements of same group (ia). three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. As. Element X Y Z Have Atomic Number 19 37 55.
From mybios.me
Periodic Table Protons Neutrons And Electrons List Bios Pics Element X Y Z Have Atomic Number 19 37 55 Which of the following statements is false about. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x'. Element X Y Z Have Atomic Number 19 37 55.
From utedzz.blogspot.com
Periodic Table With Protons Electrons And Neutrons Periodic Table Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Which of the following statements is false about. Within a group, ie decreases from top. Element X Y Z Have Atomic Number 19 37 55.
From www.sliderbase.com
The Atom Presentation Chemistry Element X Y Z Have Atomic Number 19 37 55 (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie. Element X Y Z Have Atomic Number 19 37 55.
From www.geeksforgeeks.org
Atomic Mass Table of First 30 Elements Element X Y Z Have Atomic Number 19 37 55 Within a group, ie decreases from top to bottom. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would. Element X Y Z Have Atomic Number 19 37 55.
From chemistry.about.com
Element List Atomic Number, Element Name and Symbol Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Within a group, ie decreases from top to bottom. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19),. Element X Y Z Have Atomic Number 19 37 55.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. Within a group, ie decreases from top to bottom. It is given that x, y and z have atomic numbers 19,37 and 55 respectively. three elements x , y. Element X Y Z Have Atomic Number 19 37 55.
From periodictable.me
Printable Periodic table with atomic number Dynamic Periodic Table of Element X Y Z Have Atomic Number 19 37 55 elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). The elements x (19), y (37) and z (55) are the elements of same group (ia). (a) their ionization potential would increases with increasing atomic number ( b. Element X Y Z Have Atomic Number 19 37 55.
From freehomedelivery.net
Periodic Table Periodic Table Of Elements, Table Of Elements, Modern Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). It is given that x, y and z have atomic. Element X Y Z Have Atomic Number 19 37 55.
From primavse.weebly.com
Printable periodic table of elements with atomic mass primavse Element X Y Z Have Atomic Number 19 37 55 elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. Within a group, ie decreases from top to bottom. The elements x (19),. Element X Y Z Have Atomic Number 19 37 55.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. three elements x , y and z have atomic numbers. Element X Y Z Have Atomic Number 19 37 55.
From sciencenotes.org
What Are the First 20 Elements Names and Symbols Element X Y Z Have Atomic Number 19 37 55 Within a group, ie decreases from top to bottom. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. The elements x (19), y (37) and z (55) are the elements of same group (ia). As. Element X Y Z Have Atomic Number 19 37 55.
From revisionscience.com
Atomic Number and Mass Number ALevel Chemistry Element X Y Z Have Atomic Number 19 37 55 The elements x (19), y (37) and z (55) are the elements of same group (ia). elements x, y and z with atomic numbers 19, 37, 55 lie in group 1 (alkali metals). (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z'. Element X Y Z Have Atomic Number 19 37 55.
From periodictable.me
Modern Periodic Table of Elements with Names and Symbols Element X Y Z Have Atomic Number 19 37 55 (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an ionization potential between those of 'x' and 'z' ( c ) 'z' would have the highest. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Within a group, ie decreases from top to bottom.. Element X Y Z Have Atomic Number 19 37 55.
From www.walmart.com
Periodic Table Science Chart 2021 LAMINATED Classroom Poster 15x20 Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). Within a group, ie decreases from top to bottom. (a) their ionization potential would increases with increasing atomic number ( b ) 'y' would have an. Element X Y Z Have Atomic Number 19 37 55.
From sgwebdigital.com
Chemische elementen Atomen SG Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. Which of the following statements is false about. As atomic number, increases, the ionization. three elements x , y and z have atomic numbers 19 , 37. Element X Y Z Have Atomic Number 19 37 55.
From depositphotos.com
Periodic Table Elements Shows Atomic Number Symbol Name Atomic Weight Element X Y Z Have Atomic Number 19 37 55 It is given that x, y and z have atomic numbers 19,37 and 55 respectively. Within a group, ie decreases from top to bottom. As atomic number, increases, the ionization. The elements x (19), y (37) and z (55) are the elements of same group (ia). three elements x , y and z have atomic numbers 19 , 37. Element X Y Z Have Atomic Number 19 37 55.
From cabinet.matttroy.net
Periodic Table Of Elements Names And Symbols List In Order Pdf Element X Y Z Have Atomic Number 19 37 55 three elements x , y and z have atomic numbers 19 , 37 and 55 respectively. The elements x (19), y (37) and z (55) are the elements of same group (ia). elements ′x′,′y ′ and ′z′ have atomic numbers 19,37 and 55 respectively. Within a group, ie decreases from top to bottom. As atomic number, increases, the. Element X Y Z Have Atomic Number 19 37 55.