Dilution Old Definition . dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration. Learn how to dilute and concentrate solutions. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of. state whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms:
from microbeonline.com
dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration. Concentration is the removal of. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
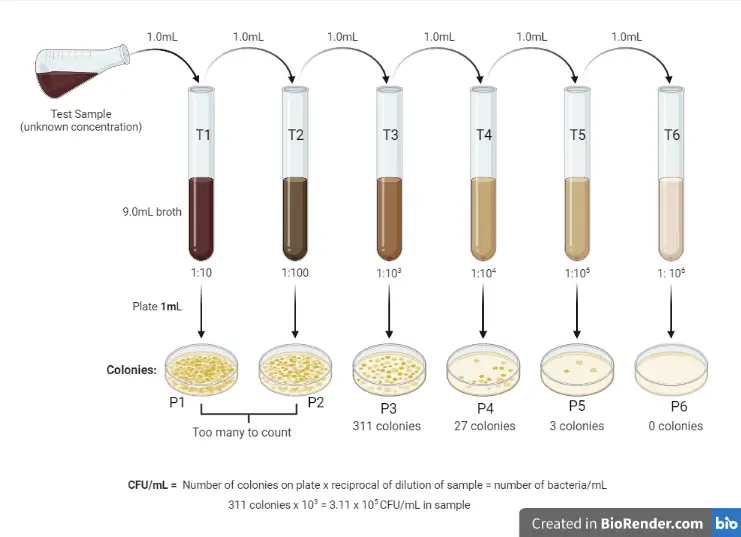
Serial Dilution Method for Estimating Viable Count of Bacteria
Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. Concentration is the removal of solvent, which increases the concentration. Learn how to dilute and concentrate solutions. we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to reducing the concentration of a solute in a. Dilution Old Definition.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Old Definition Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to reducing the concentration of a solute in a solution, usually by. Dilution Old Definition.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Old Definition state whether the concentration of a solution is directly or indirectly proportional to its volume. we will begin our discussion of solution concentration with two related and relative terms: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution. Dilution Old Definition.
From www.youtube.com
DILUTIONDEFINITION AND PRACTICE PROBLEMS YouTube Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution refers to reducing the concentration of a solute in a solution, usually by adding more. Dilution Old Definition.
From fabrikbrands.com
What Is Brand Dilution? Brand Dilution Definition With Examples Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: Learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. state whether the concentration of a solution. Dilution Old Definition.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the. Dilution Old Definition.
From ceqnefyw.blob.core.windows.net
Dilution Method Of Ast at Clement Meador blog Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and. Dilution Old Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Old Definition Concentration is the removal of. Learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. we will begin our discussion of solution. Dilution Old Definition.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. we will begin our discussion of solution concentration. Dilution Old Definition.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria Dilution Old Definition Often, a worker will need to change the concentration of a solution by. we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration. Learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Old Definition.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution. Dilution Old Definition.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. state whether the concentration of a solution. Dilution Old Definition.
From www.aquaportail.com
Dilution définition et explications Dilution Old Definition state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which increases the concentration. dilution. Dilution Old Definition.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of.. Dilution Old Definition.
From ceqnefyw.blob.core.windows.net
Dilution Method Of Ast at Clement Meador blog Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of. Dilution Old Definition.
From cepdqvxp.blob.core.windows.net
Dilute Solution Definition Chemistry at Elizabeth Knudson blog Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. Often, a worker will. Dilution Old Definition.
From slideplayer.com
Proportional Relationships ppt download Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the. Dilution Old Definition.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Old Definition dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of. Often, a worker will need to change the concentration of a solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases. Dilution Old Definition.
From thestudentnotes.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses The Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Old Definition.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Old Definition dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. we will begin our discussion of solution concentration with two related and relative terms: state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration. Dilution Old Definition.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the addition of solvent, which decreases the concentration. Dilution Old Definition.
From opentrons.com
Serial Dilution Guide for Laboratory Sciences Dilution Old Definition dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Learn how to dilute and concentrate solutions. Concentration is the removal of. Often, a worker will need to change the concentration of a solution by. we will begin our discussion of solution concentration with two related and relative terms: Concentration is. Dilution Old Definition.
From exoyaaeul.blob.core.windows.net
Dilution Definition In A Sentence at Tomas Branson blog Dilution Old Definition Concentration is the removal of. state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related. Dilution Old Definition.
From mungfali.com
10 Fold Serial Dilution Dilution Old Definition we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. state whether the concentration of a solution is directly or indirectly proportional to. Dilution Old Definition.
From cexawjgq.blob.core.windows.net
What Is The Purpose Of Dilution Assay at Walter Harrison blog Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. Often, a worker will need to change the concentration of a solution by. Learn how to dilute and concentrate solutions. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of. dilution is the addition of solvent,. Dilution Old Definition.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. Concentration is the removal of. Often, a worker will need to change the concentration of a solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. we will begin our discussion of solution concentration with two related and relative terms: . Dilution Old Definition.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Old Definition Often, a worker will need to change the concentration of a solution by. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. we will begin our discussion of solution concentration with two related and relative terms: dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Old Definition.
From ceqdayvh.blob.core.windows.net
Serial Dilution In Microbiology Ppt at Roxane Luoma blog Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of solvent, which increases the concentration. Concentration is. Dilution Old Definition.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. dilution refers to reducing the concentration. Dilution Old Definition.
From www.youtube.com
Serial Dilution Required Practical Revision for Biology and Chemistry Dilution Old Definition Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. Dilution Old Definition.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Dilution Old Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration. dilution refers to reducing the concentration of a solute in a solution, usually by adding. Dilution Old Definition.
From www.fool.com
Understanding Stock Dilution and Why You Should Care About It The Dilution Old Definition Concentration is the removal of solvent, which increases the concentration. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Often, a worker will need to change the concentration of a solution by. Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly. Dilution Old Definition.
From stock.adobe.com
The tenfold serial dilution of pathogen suspension in solution sample Dilution Old Definition Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the. Dilution Old Definition.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Old Definition Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases. Dilution Old Definition.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Old Definition Often, a worker will need to change the concentration of a solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution refers to reducing the concentration of a solute in a solution, usually by adding more solvent. Learn how to dilute and concentrate solutions. Concentration is the removal of solvent,. Dilution Old Definition.