Standard Enthalpy Of Formation Solve . For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. The key to solving this problem is to have a table of standard enthalpies of formation handy. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Each element must be in the. In case you missed it, look at the equation up near the. Characteristics of enthalpy of formation. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition.
from www.chegg.com
For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. In case you missed it, look at the equation up near the. Each element must be in the. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The key to solving this problem is to have a table of standard enthalpies of formation handy. The standard enthalpy of formation of any element in its standard state is zero by definition. Characteristics of enthalpy of formation. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
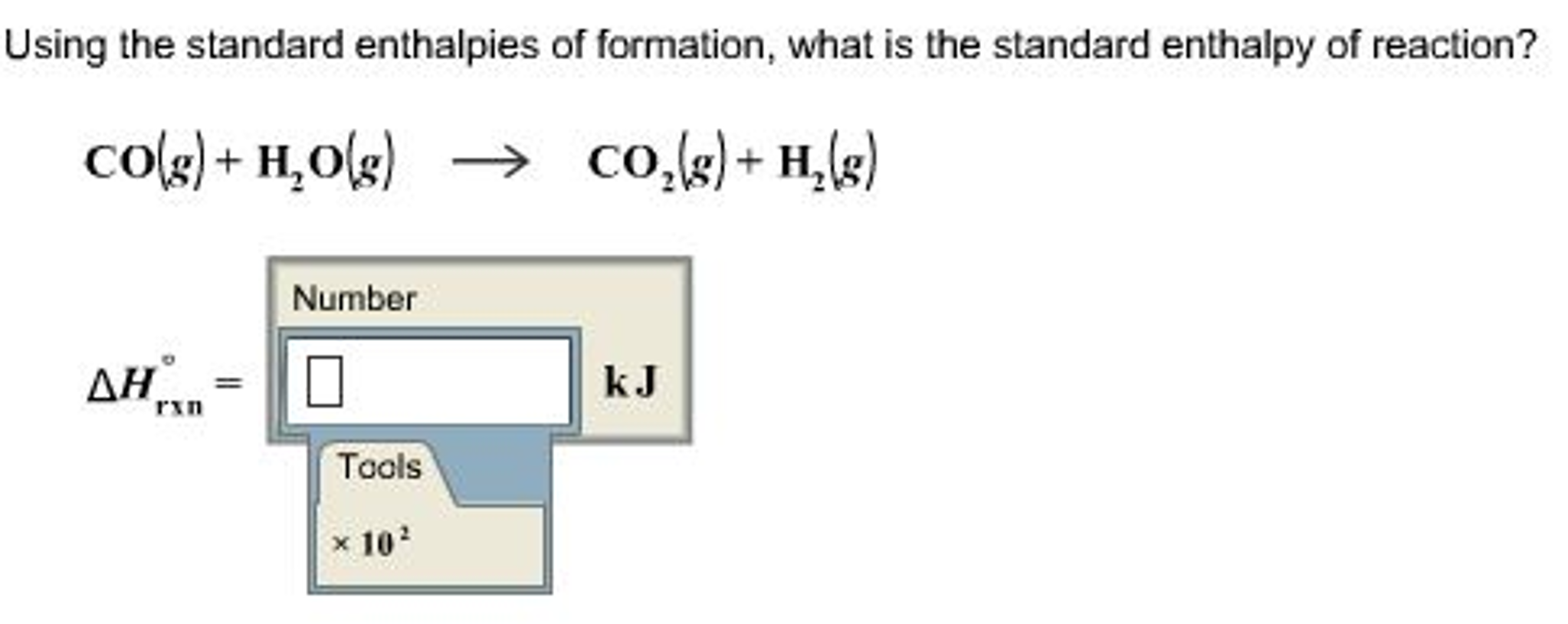
Solved Using the standard enthalpies of formation, what is
Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. In case you missed it, look at the equation up near the. Characteristics of enthalpy of formation. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The key to solving this problem is to have a table of standard enthalpies of formation handy. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Each element must be in the. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.chegg.com
Solved The standard enthalpies of formation for several Standard Enthalpy Of Formation Solve For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Characteristics of enthalpy of formation. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Solve Characteristics of enthalpy of formation. The key to solving this problem is to have a table of standard enthalpies of formation handy. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED The standard enthalpy of combustion of ethene gas, C₂H4 (g), is Standard Enthalpy Of Formation Solve The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Of Formation Solve The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. In case. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation and the standard Standard Enthalpy Of Formation Solve Characteristics of enthalpy of formation. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. In case you missed it, look at the equation up near the. The key to solving this problem is to have a table of standard enthalpies of formation handy.. Standard Enthalpy Of Formation Solve.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. Standard enthalpy of. Standard Enthalpy Of Formation Solve.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Solve Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation, also known as the heat of formation, is the. Standard Enthalpy Of Formation Solve.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Solve Each element must be in the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The. Standard Enthalpy Of Formation Solve.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Solve The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. In. Standard Enthalpy Of Formation Solve.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Solve The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The key to solving this problem is to. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Solve Characteristics of enthalpy of formation. The key to solving this problem is to have a table of standard enthalpies of formation handy. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Each element must be in the. 193 rows in chemistry and thermodynamics, the. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Solve Each element must be in the. Characteristics of enthalpy of formation. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Characteristics of enthalpy of formation. Each element must be in the. The standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Using the standard enthalpies of formation, what is Standard Enthalpy Of Formation Solve Each element must be in the. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Using the standard enthalpies of formation found in the Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Characteristics of enthalpy of formation. Standard enthalpy of formation (or. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Enthalpy of formation of phenol (C6H5OH) is 165 kJ/mol , and Standard Enthalpy Of Formation Solve The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is the enthalpy. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Standard Enthalpy Of Formation Solve Each element must be in the. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the reaction at 25 ∘ Standard Enthalpy Of Formation Solve Characteristics of enthalpy of formation. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Each element must be in the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. The key to solving this problem is to have a table of standard enthalpies of formation handy. For example, although oxygen. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of CH3OH(I) from Standard Enthalpy Of Formation Solve Each element must be in the. Characteristics of enthalpy of formation. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Using the standard enthalpies of formation, what is Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Each element must be in the. Characteristics. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Solve Each element must be in the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. In case you missed it, look at the equation up near the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Enthalpy Of Formation Solve.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Solve Each element must be in the. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. The key to solving this problem is to have a table of standard enthalpies of formation handy. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. The standard enthalpy of formation is defined as the. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved Which processes in the table have enthalpies of Standard Enthalpy Of Formation Solve In case you missed it, look at the equation up near the. Each element must be in the. The key to solving this problem is to have a table of standard enthalpies of formation handy. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. Standard Enthalpy Of Formation Solve.
From www.studypool.com
SOLUTION 5 CALCULATION OF ENTHALPY CHANGES OF REACTIONS USING Standard Enthalpy Of Formation Solve Characteristics of enthalpy of formation. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation $δh°_f$ is an enthalpy. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved A scientist measures the standard enthalpy change for Standard Enthalpy Of Formation Solve The standard enthalpy of formation of any element in its standard state is zero by definition. Each element must be in the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Characteristics of enthalpy of formation. Standard enthalpy of formation (or heat of. Standard Enthalpy Of Formation Solve.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Solve The key to solving this problem is to have a table of standard enthalpies of formation handy. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Each element must be in the. Characteristics of enthalpy of formation. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Solve.
From www.coursehero.com
[Solved] Which of the substances have a standard enthalpy of formation Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The key to solving this problem is to have a table of standard enthalpies of formation handy. In case you missed it, look at the equation up near the. The standard enthalpy of formation is the enthalpy. Standard Enthalpy Of Formation Solve.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Solve Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved 15. Using standard enthalpies of formation, calculate Standard Enthalpy Of Formation Solve The key to solving this problem is to have a table of standard enthalpies of formation handy. In case you missed it, look at the equation up near the. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193. Standard Enthalpy Of Formation Solve.
From www.chegg.com
Solved You are given the following standard enthalpies of Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Characteristics of enthalpy of formation. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The key. Standard Enthalpy Of Formation Solve.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Solve 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Solve.
From www.numerade.com
SOLVED Exercise EZC.2(a) The standard enthalpy of formation of Standard Enthalpy Of Formation Solve The key to solving this problem is to have a table of standard enthalpies of formation handy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation Solve.