What Intermolecular Forces Does Bromine Have . The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. And iodine, i_2, is a volatile,. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Find out the names of the forces. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Bromine, br_2, is a room temperature liquid. It is unlikely to be a solid at room temperature. Intermolecular forces (imfs) can be used to predict relative boiling points. There are three types of intermolecular forces:
from www.slideserve.com
There are three types of intermolecular forces: The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. Bromine, br_2, is a room temperature liquid. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. And iodine, i_2, is a volatile,. It is unlikely to be a solid at room temperature. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points.
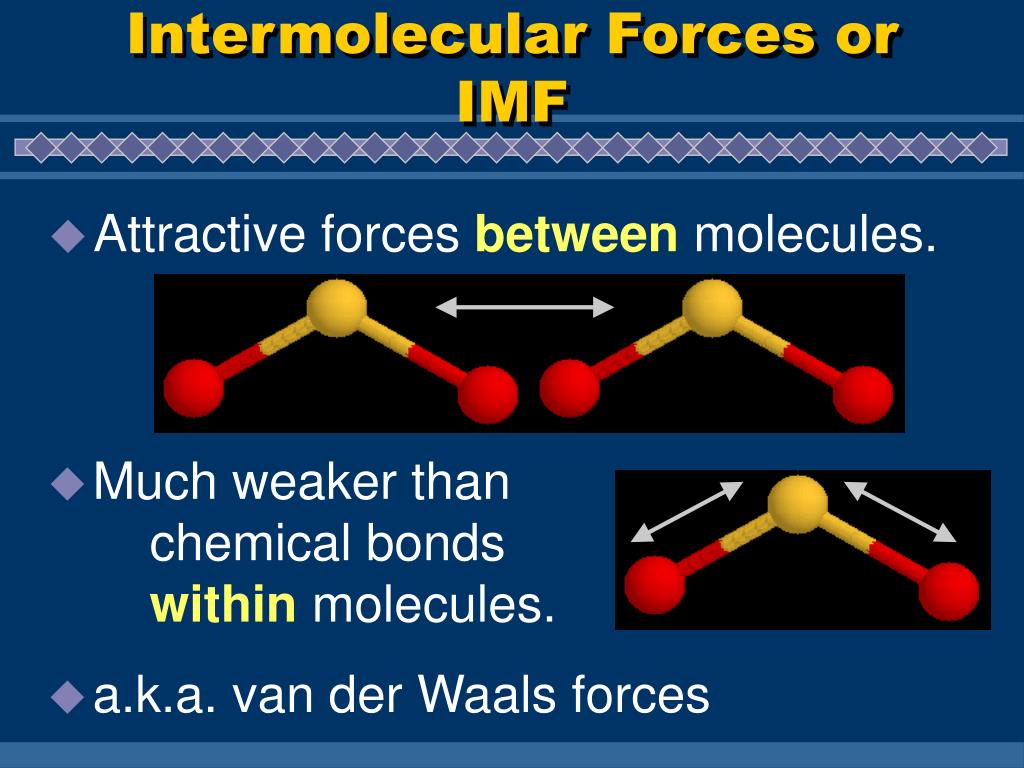
PPT Chapter 6 4 Intermolecular Forces or IMF (p. 219 224
What Intermolecular Forces Does Bromine Have The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Find out the names of the forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. There are three types of intermolecular forces: And iodine, i_2, is a volatile,. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. It is unlikely to be a solid at room temperature. Bromine, br_2, is a room temperature liquid. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces (imfs) can be used to predict relative boiling points.
From slideplayer.com
Intermolecular Forces ppt download What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Find out the names of the forces. And iodine, i_2, is a volatile,. There are. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Chapter 6 4 Intermolecular Forces or IMF (p. 219 224 What Intermolecular Forces Does Bromine Have Find out the names of the forces. Bromine, br_2, is a room temperature liquid. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. And iodine, i_2, is a volatile,. It is unlikely to be a solid at room temperature. It is unlikely to. What Intermolecular Forces Does Bromine Have.
From www.numerade.com
In addition to dispersion forces, what intermolecular forces are What Intermolecular Forces Does Bromine Have Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Intermolecular forces (imfs) can be used to predict relative boiling points. And iodine, i_2, is a volatile,. It is unlikely to be a solid at room temperature. Find out the names of the forces. The stronger the imfs, the lower the vapor pressure of the substance and. What Intermolecular Forces Does Bromine Have.
From slideplayer.com
Intermolecular Forces ppt download What Intermolecular Forces Does Bromine Have Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. There are three types of intermolecular forces: In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Thus,. What Intermolecular Forces Does Bromine Have.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. There are three types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. And iodine, i_2, is a volatile,. Find out the names of the forces. In this video we’ll identify the intermolecular. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation What Intermolecular Forces Does Bromine Have In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. There are three types of intermolecular forces: The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. It is unlikely to be a solid at room temperature. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3). What Intermolecular Forces Does Bromine Have.
From sciencenotes.org
Intermolecular Forces in Chemistry What Intermolecular Forces Does Bromine Have The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. There are three types of intermolecular forces: Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3). What Intermolecular Forces Does Bromine Have.
From www.numerade.com
SOLVED 4) Describe the physical properties of an amine group. Are they What Intermolecular Forces Does Bromine Have Fluorine, f_2, and chlorine, cl_2 are room temperature gases. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. There are three types of intermolecular forces: Find out the names of the forces. It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. And iodine,. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Intermolecular Forces Does Bromine Have Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Bromine, br_2, is a room temperature liquid. It is unlikely to be a solid at room temperature. It is unlikely to be a solid at room temperature. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Find out the names. What Intermolecular Forces Does Bromine Have.
From gunner-kmorse.blogspot.com
Which of the Following Halogens Would Have Stronger Intermolecular Forces What Intermolecular Forces Does Bromine Have In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. There are three types of intermolecular forces: Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Intermolecular forces (imfs) can be used to predict relative boiling points. Find out the names of the forces. Bromine, br_2, is a room temperature liquid. And iodine, i_2, is a volatile,. Learn. What Intermolecular Forces Does Bromine Have.
From chem.libretexts.org
12.7 Intermolecular forces Chemistry LibreTexts What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. Intermolecular forces (imfs) can be used to predict relative boiling points. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Find out the names of the forces. There are three types of intermolecular forces: Learn how the. What Intermolecular Forces Does Bromine Have.
From slideplayer.com
Intermolecular Forces Notes ppt download What Intermolecular Forces Does Bromine Have Bromine, br_2, is a room temperature liquid. It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Intermolecular forces (imfs) can be used to predict relative boiling points. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. It is unlikely to be a solid at room temperature.. What Intermolecular Forces Does Bromine Have.
From www.numerade.com
SOLVED Draw the Lewis structures for hydrogen sulfide (H2S) and What Intermolecular Forces Does Bromine Have There are three types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. It is unlikely to be a solid at room temperature. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Intermolecular. What Intermolecular Forces Does Bromine Have.
From socratic.org
What are the intermolecular forces? + Example What Intermolecular Forces Does Bromine Have And iodine, i_2, is a volatile,. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Intermolecular forces (imfs) can be used to predict relative boiling points. Bromine, br_2, is a room temperature liquid. There are three types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular forces other than dispersion. What Intermolecular Forces Does Bromine Have.
From www.numerade.com
SOLVEDOH OH HCI Cl Cl What intermolecular forces does the above What Intermolecular Forces Does Bromine Have Intermolecular forces (imfs) can be used to predict relative boiling points. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room. What Intermolecular Forces Does Bromine Have.
From www.youtube.com
BrF Polar or Nonpolar (Bromine fluoride) YouTube What Intermolecular Forces Does Bromine Have There are three types of intermolecular forces: In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Find out the names of the forces. And iodine, i_2, is a volatile,. The stronger the imfs, the lower the vapor pressure of the substance and the higher. What Intermolecular Forces Does Bromine Have.
From techiescientist.com
HCl Intermolecular Forces — Type, Strong or Weak? Techiescientist What Intermolecular Forces Does Bromine Have Bromine, br_2, is a room temperature liquid. Find out the names of the forces. There are three types of intermolecular forces: It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. The stronger the imfs, the lower the vapor pressure of the substance and the higher the. What Intermolecular Forces Does Bromine Have.
From irajlorenzeno.blob.core.windows.net
Does Surface Tension Increase With Intermolecular Forces at What Intermolecular Forces Does Bromine Have There are three types of intermolecular forces: The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces (imfs) can be used to predict relative boiling points. Bromine, br_2, is a room temperature liquid. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. In this video we’ll identify the intermolecular forces. What Intermolecular Forces Does Bromine Have.
From jsmithmoore.com
Intermolecular forces What Intermolecular Forces Does Bromine Have Intermolecular forces (imfs) can be used to predict relative boiling points. Find out the names of the forces. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. And iodine, i_2, is a volatile,. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect. What Intermolecular Forces Does Bromine Have.
From sciencenotes.org
Intramolecular Forces What Intermolecular Forces Does Bromine Have Find out the names of the forces. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. Bromine, br_2, is a room temperature liquid. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. There are three types of. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download What Intermolecular Forces Does Bromine Have Find out the names of the forces. Bromine, br_2, is a room temperature liquid. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. It. What Intermolecular Forces Does Bromine Have.
From www.chemistrylearner.com
Intermolecular Forces Definition, Types, and Examples What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the. What Intermolecular Forces Does Bromine Have.
From ar.inspiredpencil.com
Hydrogen Bonding Intermolecular Forces What Intermolecular Forces Does Bromine Have Find out the names of the forces. Intermolecular forces (imfs) can be used to predict relative boiling points. It is unlikely to be a solid at room temperature. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. There are three types of intermolecular forces: Thus, diatomic bromine does not have any intermolecular. What Intermolecular Forces Does Bromine Have.
From www.youtube.com
Intermolecular Forces for O2 (Molecular Oxygen / Diatomic Oxygen) YouTube What Intermolecular Forces Does Bromine Have And iodine, i_2, is a volatile,. Bromine, br_2, is a room temperature liquid. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature. Find out the names of the forces. There are three types of. What Intermolecular Forces Does Bromine Have.
From brainybreeze.blue
Nitrogen Trichloride Intermolecular Forces Brainy Breeze What Intermolecular Forces Does Bromine Have Bromine, br_2, is a room temperature liquid. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. It is unlikely to be a. What Intermolecular Forces Does Bromine Have.
From scienceinfo.com
Intermolecular Forces Definition and Types What Intermolecular Forces Does Bromine Have The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. It is unlikely to be a solid at room temperature. Bromine, br_2, is a room temperature. What Intermolecular Forces Does Bromine Have.
From www.youtube.com
How to identify intermolecular forces? YouTube What Intermolecular Forces Does Bromine Have And iodine, i_2, is a volatile,. Find out the names of the forces. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. It is unlikely to be a solid at room temperature. It is unlikely to be a solid at room temperature. There are three types of. What Intermolecular Forces Does Bromine Have.
From www.slideshare.net
Intermolecular forces What Intermolecular Forces Does Bromine Have Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. There are three types of intermolecular forces: The stronger the imfs, the lower the vapor pressure of. What Intermolecular Forces Does Bromine Have.
From studiousguy.com
Intermolecular Force Types and Examples StudiousGuy What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. And iodine, i_2, is a volatile,. There are three types of intermolecular forces: Thus, diatomic. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Monday January 3 , 2011 PowerPoint Presentation, free download What Intermolecular Forces Does Bromine Have Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Find out the names of the forces. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. The stronger the imfs, the lower the vapor pressure of the substance and the higher. What Intermolecular Forces Does Bromine Have.
From sciencenotes.org
Intermolecular Forces in Chemistry What Intermolecular Forces Does Bromine Have It is unlikely to be a solid at room temperature. Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. There are three types of intermolecular forces: Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Intermolecular forces (imfs) can be used to predict relative boiling points. Find out the names of the forces. It is unlikely. What Intermolecular Forces Does Bromine Have.
From www.numerade.com
SOLVED Which answer best describes the strongest intermolecular force What Intermolecular Forces Does Bromine Have Bromine, br_2, is a room temperature liquid. It is unlikely to be a solid at room temperature. There are three types of intermolecular forces: It is unlikely to be a solid at room temperature. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Find out the names of the forces. The stronger the imfs, the lower the. What Intermolecular Forces Does Bromine Have.
From www.chegg.com
Solved Question 5 What is the strongest intermolecular force What Intermolecular Forces Does Bromine Have Thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. Thus, diatomic bromine does not have any intermolecular forces. What Intermolecular Forces Does Bromine Have.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID3151726 What Intermolecular Forces Does Bromine Have Fluorine, f_2, and chlorine, cl_2 are room temperature gases. Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Thus, diatomic bromine does. What Intermolecular Forces Does Bromine Have.
From www.numerade.com
SOLVEDQuestion 9 (1 point) What types of intermolecular forces does What Intermolecular Forces Does Bromine Have Learn how the intermolecular forces of fluorine (f2), bromine (br2) and ammonia (nh3) affect their boiling points. And iodine, i_2, is a volatile,. Fluorine, f_2, and chlorine, cl_2 are room temperature gases. It is unlikely to be a solid at room temperature. Find out the names of the forces. It is unlikely to be a solid at room temperature. Thus,. What Intermolecular Forces Does Bromine Have.