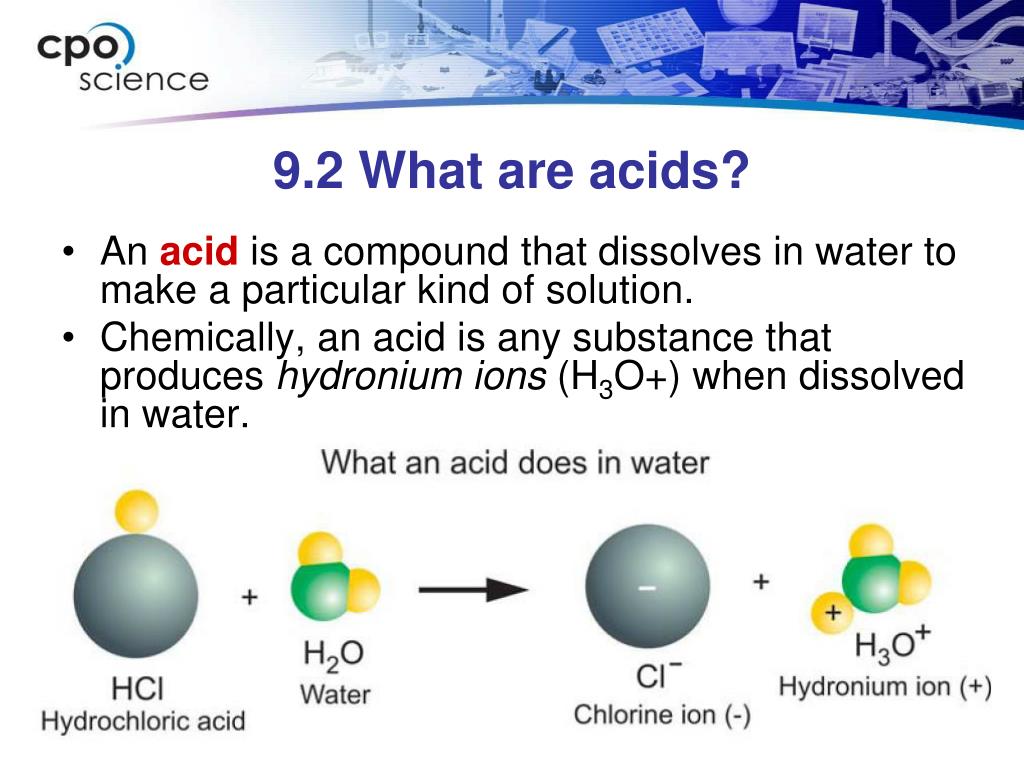
What Occurs When You Add An Acid To A Base . According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. See examples of common acids and bases, their strengths, and how they neutralize. Learn how acids and bases react with water and each other to form salts and water. This keeps the ph relatively stable. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas.
from www.slideserve.com
According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. This keeps the ph relatively stable. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. Learn how acids and bases react with water and each other to form salts and water. See examples of common acids and bases, their strengths, and how they neutralize. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. It can be used to determine ph via titration.
PPT 9.2 What are acids? PowerPoint Presentation, free download ID
What Occurs When You Add An Acid To A Base According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. It can be used to determine ph via titration. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react with water and each other to form salts and water. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby.
From materialschoolsydney.z13.web.core.windows.net
Acid Base Neutralization Reactions Worksheet What Occurs When You Add An Acid To A Base See examples of common acids and bases, their strengths, and how they neutralize. Learn how acids and bases react with water and each other to form salts and water. This keeps the ph relatively stable. It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with. What Occurs When You Add An Acid To A Base.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Occurs When You Add An Acid To A Base This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. It can be used to determine ph via titration. When you add an acid or base to the buffer, the. What Occurs When You Add An Acid To A Base.
From sciencenotes.org
Household Acids and Bases What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. Learn how acids and bases react with water and each other to form salts and water. See examples. What Occurs When You Add An Acid To A Base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in. What Occurs When You Add An Acid To A Base.
From www.teachoo.com
Assertion (A) To dilute sulphuric acid, acid is added to water and n What Occurs When You Add An Acid To A Base This keeps the ph relatively stable. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby.. What Occurs When You Add An Acid To A Base.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in. What Occurs When You Add An Acid To A Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Occurs When You Add An Acid To A Base When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. See examples of common acids and bases, their strengths, and how they neutralize. This keeps the ph relatively stable. It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how. What Occurs When You Add An Acid To A Base.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph. What Occurs When You Add An Acid To A Base.
From go.isptutor.org
Chemical vs. Physical Reactions/Processes What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. Learn how acids and bases react with water and each other to form salts and water. When you add an acid or base to the buffer, the buffer components react to neutralize. What Occurs When You Add An Acid To A Base.
From www.teachoo.com
Why do HCl, HNO3, etc., show acidic characters in aqueous solutions What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. According to brønsted and. What Occurs When You Add An Acid To A Base.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler What Occurs When You Add An Acid To A Base This keeps the ph relatively stable. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. It can be used to determine ph via titration. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\). What Occurs When You Add An Acid To A Base.
From www.youtube.com
Reactions of Acids and Bases YouTube What Occurs When You Add An Acid To A Base Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form. What Occurs When You Add An Acid To A Base.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Occurs When You Add An Acid To A Base Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. See examples of common acids and bases, their strengths, and how they neutralize. This keeps the ph relatively stable. According. What Occurs When You Add An Acid To A Base.
From www.slideshare.net
Presentation Acids What Occurs When You Add An Acid To A Base When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. Learn how acids and bases react with water and each other to form salts and water. This keeps the ph. What Occurs When You Add An Acid To A Base.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary What Occurs When You Add An Acid To A Base Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph relatively stable. It can be used to determine ph via titration. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an. What Occurs When You Add An Acid To A Base.
From www.chemistrysteps.com
Alcohol Reaction with HCl, HBr and HI Acids Chemistry Steps What Occurs When You Add An Acid To A Base When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. See examples of common acids and bases, their. What Occurs When You Add An Acid To A Base.
From www.tes.com
Chemistry GCSE 91 Reactions of Acids Teaching Resources What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \. What Occurs When You Add An Acid To A Base.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. See examples of common acids and bases, their strengths, and how they neutralize. It can be used to determine ph via titration. This keeps the. What Occurs When You Add An Acid To A Base.
From www.slideshare.net
Acids & Bases What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. It can be used to determine ph via titration. See examples of common acids and bases, their strengths, and how they neutralize. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion. What Occurs When You Add An Acid To A Base.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution,. What Occurs When You Add An Acid To A Base.
From www.youtube.com
Identifying Acids, Bases, & Conjugate Acids & Bases in an Acid/Base What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. This keeps the ph relatively stable. See examples of common acids. What Occurs When You Add An Acid To A Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. This keeps the ph relatively stable. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. See examples of common acids and bases, their strengths, and how they neutralize. When you add an acid or base to the buffer, the. What Occurs When You Add An Acid To A Base.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph relatively stable. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. According to brønsted and lowry,. What Occurs When You Add An Acid To A Base.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and What Occurs When You Add An Acid To A Base It can be used to determine ph via titration. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react with water and each other. What Occurs When You Add An Acid To A Base.
From scienceandsf.com
May 2020 Scienceandsf A Blog Published by Robert A. Lawler What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. When you add an acid or base to the buffer, the. What Occurs When You Add An Acid To A Base.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. This keeps the ph relatively stable. It can be used to determine ph via titration. According to brønsted and lowry, an acid (a substance with. What Occurs When You Add An Acid To A Base.
From www.slideserve.com
PPT Page 12 Physical Property A property that can be observed with What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. See examples of common acids and bases, their strengths, and how. What Occurs When You Add An Acid To A Base.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations What Occurs When You Add An Acid To A Base According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph relatively stable.. What Occurs When You Add An Acid To A Base.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs What Occurs When You Add An Acid To A Base According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react to form. What Occurs When You Add An Acid To A Base.
From www.slideserve.com
PPT Jumpstart PowerPoint Presentation, free download ID2375027 What Occurs When You Add An Acid To A Base This keeps the ph relatively stable. Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. It can be used to determine ph via titration. See examples of common acids. What Occurs When You Add An Acid To A Base.
From www.online-sciences.com
Classification of Acids according to its strength (degree of ionization What Occurs When You Add An Acid To A Base According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous solution, thereby. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Learn how acids and bases react to form. What Occurs When You Add An Acid To A Base.
From mchsscience.weebly.com
Acids and Bases MCHS Science What Occurs When You Add An Acid To A Base Learn how acids and bases react to form salts and water, and how they react with metals to produce hydrogen gas. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. According. What Occurs When You Add An Acid To A Base.
From www.youtube.com
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. This keeps the ph relatively stable. See examples of common acids and bases, their strengths, and how they neutralize. It can be used to determine ph via titration. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that. What Occurs When You Add An Acid To A Base.
From lessonlibrarysnotter.z13.web.core.windows.net
Identify The Neutralization Reaction What Occurs When You Add An Acid To A Base Learn how acids and bases react with water and each other to form salts and water. See examples of common acids and bases, their strengths, and how they neutralize. According to brønsted and lowry, an acid (a substance with at least one hydrogen atom that can dissociate to form an anion and an \ (h^+\) ion (a proton) in aqueous. What Occurs When You Add An Acid To A Base.
From www.slideserve.com
PPT 9.2 What are acids? PowerPoint Presentation, free download ID What Occurs When You Add An Acid To A Base When you add an acid or base to the buffer, the buffer components react to neutralize the added substance. It can be used to determine ph via titration. Learn how acids and bases react with water and each other to form salts and water. See examples of common acids and bases, their strengths, and how they neutralize. Learn how acids. What Occurs When You Add An Acid To A Base.