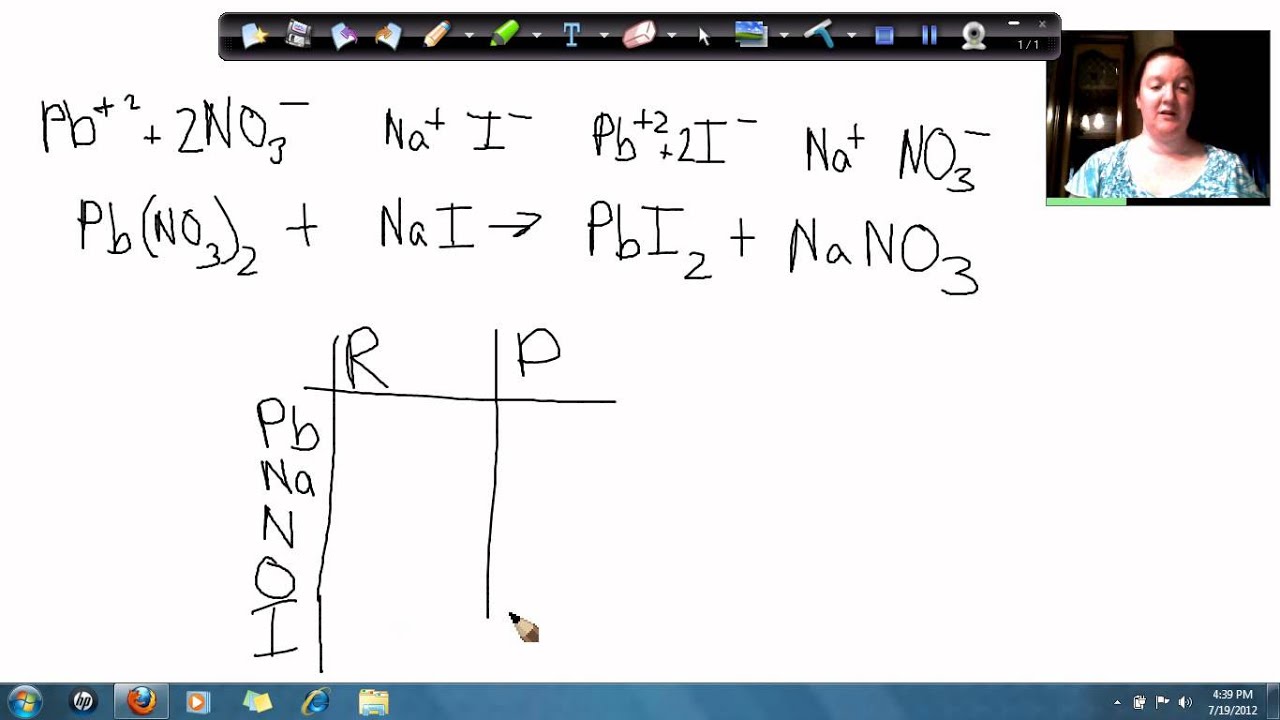
Lead Chloride Plus Water . this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. It is very slightly soluble in cold water, but more soluble in hot water. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Because many lead (ii) compounds. lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature.
from ceryouwh.blob.core.windows.net
it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. It is very slightly soluble in cold water, but more soluble in hot water. lead (ii) chloride is a white solid, melting at 501°c. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. It is slightly soluble in cold water, but. Because many lead (ii) compounds. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the.
Lead Nitrate + Sodium Chloride Gives at Joseph Fife blog
Lead Chloride Plus Water lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Because many lead (ii) compounds. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It is very slightly soluble in cold water, but more soluble in hot water. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but.
From www.coursehero.com
[Solved] Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Chloride Plus Water it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. lead (ii) chloride is a white solid, melting at 501°c. lead(ii) ion. Lead Chloride Plus Water.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Lead Chloride Plus Water lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. Because many. Lead Chloride Plus Water.
From www.fishersci.pt
Lead(II) chloride, 99, Thermo Scientific Chemicals Fisher Scientific Lead Chloride Plus Water in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. It is slightly soluble in cold water, but. lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Because many lead (ii) compounds.. Lead Chloride Plus Water.
From www.ottokemi.com
Cotunnite, Plumbous chloride, Lead dichloride Lead(II) chloride, 99 Lead Chloride Plus Water lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but. it. Lead Chloride Plus Water.
From fphoto.photoshelter.com
science chemistry precipitation reaction nickel chloride Fundamental Lead Chloride Plus Water It is very slightly soluble in cold water, but more soluble in hot water. Because many lead (ii) compounds. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. lead(ii) ion. Lead Chloride Plus Water.
From www.numerade.com
SOLVED Given 350 mL of 3.2 M Pb(NO3)2 and 200 mL of 0.02 M NaCl are Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Because many lead (ii) compounds. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but. in this. Lead Chloride Plus Water.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Lead Chloride Plus Water this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Because many lead (ii) compounds. It is very. Lead Chloride Plus Water.
From www.youtube.com
CaCl2 + H2O (Calcium chloride + Water) YouTube Lead Chloride Plus Water it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead (ii) chloride is a white solid, melting at 501°c. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. Unlike the tetrachlorides, lead(ii) chloride can be considered. Lead Chloride Plus Water.
From www.youtube.com
How to Balance Pb(NO3)2 + CuCl2 = PbCl2 + Cu(NO3)2 Lead (II) nitrate Lead Chloride Plus Water this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It is slightly soluble in cold water, but. It is very slightly soluble in cold water, but more soluble in hot water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate.. Lead Chloride Plus Water.
From study.com
Hydrogen Chloride vs. Hydrochloric Acid Formula, Properties Lead Chloride Plus Water it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is. Lead Chloride Plus Water.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Plus Water It is very slightly soluble in cold water, but more soluble in hot water. Because many lead (ii) compounds. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. It is slightly soluble in cold. Lead Chloride Plus Water.
From www.youtube.com
Sodium chloride in water YouTube Lead Chloride Plus Water It is slightly soluble in cold water, but. Because many lead (ii) compounds. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii). Lead Chloride Plus Water.
From www.indiamart.com
Lead Chloride powder, For Industrial, Packaging Type 50 Kg Hdpe Bag at Lead Chloride Plus Water It is slightly soluble in cold water, but. It is very slightly soluble in cold water, but more soluble in hot water. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Because many lead (ii) compounds. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. this page looks at. Lead Chloride Plus Water.
From www.bigstockphoto.com
Test Chloride Image & Photo (Free Trial) Bigstock Lead Chloride Plus Water lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but. Because many lead (ii) compounds. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is. Lead Chloride Plus Water.
From www.indiamart.com
Lead Chloride Lead Chloride Powder Manufacturer from Meerut Lead Chloride Plus Water lead (ii) chloride is a white solid, melting at 501°c. Because many lead (ii) compounds. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. It is slightly soluble in cold water, but. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h. Lead Chloride Plus Water.
From www.chegg.com
Solved The Ksp for lead chloride (PbCl2) is 1.6×10−5. Lead Chloride Plus Water It is slightly soluble in cold water, but. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. It is very slightly soluble in cold water, but more soluble in hot water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and. Lead Chloride Plus Water.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Lead Chloride Plus Water this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when.. Lead Chloride Plus Water.
From www.waterrf.org
Chloride to Sulfate Mass Ratio (CSMR) Changes from Water Treatment and Lead Chloride Plus Water Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. It is very slightly soluble in cold water, but more soluble in hot water. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. it describes the formation of lead (ii) hydroxide, lead (ii). Lead Chloride Plus Water.
From www.youtube.com
Silver Nitrate + Sodium Chloride reaction YouTube Lead Chloride Plus Water this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead (ii) chloride is a white solid, melting at 501°c. Because many lead (ii) compounds. in this video we will. Lead Chloride Plus Water.
From engineering.purdue.edu
JTRP Interaction of Chloridebased Deicing Salts with Concrete (cont. C) Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. It is very slightly soluble in cold water, but more soluble in hot water. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt,. Lead Chloride Plus Water.
From www.dreamstime.com
How Does Sodium Chloride NaCl Dissolve in Water Stock Vector Lead Chloride Plus Water Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Because many lead. Lead Chloride Plus Water.
From www.carolina.com
Lead (II) Chloride, Reagent Grade, 100 g Lead Chloride Plus Water this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It is very slightly soluble in cold water, but more soluble in hot water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to. Lead Chloride Plus Water.
From fity.club
Naoh Nh4cl Sodium Hydroxide And Ammonium Chloride Gas Lead Chloride Plus Water lead (ii) chloride is a white solid, melting at 501°c. Because many lead (ii) compounds. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is slightly soluble in cold water, but. Unlike. Lead Chloride Plus Water.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions Lead Chloride Plus Water It is slightly soluble in cold water, but. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead (ii) chloride is a white solid, melting at 501°c. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when.. Lead Chloride Plus Water.
From www.indiamart.com
Lead Chloride at best price in Mumbai by Yogi Dye Chem Industries ID Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. It is very slightly soluble in cold water, but more soluble in hot water. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. It is slightly soluble in cold water, but. Because many lead (ii) compounds.. Lead Chloride Plus Water.
From ceryouwh.blob.core.windows.net
Lead Nitrate + Sodium Chloride Gives at Joseph Fife blog Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. It is slightly soluble in cold water, but. lead (ii) chloride is a white solid, melting at 501°c. in this video we will describe the equation pbcl2 + h2o and write. Lead Chloride Plus Water.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. Because many lead (ii) compounds. lead (ii) chloride is a white solid, melting at 501°c. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is slightly soluble in cold water, but. Pbo 2 + 4 hcl →. Lead Chloride Plus Water.
From www.youtube.com
13.1.2 Describe the reactions of chlorine and the chlorides referred to Lead Chloride Plus Water Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. It is slightly soluble in cold water, but. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. this page looks at the formation of. Lead Chloride Plus Water.
From exogwagyf.blob.core.windows.net
Chlorine And Acid at Charles Borkowski blog Lead Chloride Plus Water in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. It is slightly soluble in cold water, but. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2. Lead Chloride Plus Water.
From www.dreamstime.com
PbCl4 Lead(IV) Chloride CAS 13463304 Chemical Substance in White Lead Chloride Plus Water Because many lead (ii) compounds. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. It is slightly soluble in cold water, but. lead (ii) chloride is a white solid, melting at 501°c. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.. Lead Chloride Plus Water.
From www.chegg.com
Solved How many grams of lead chloride are produced from Lead Chloride Plus Water lead (ii) chloride is a white solid, melting at 501°c. Because many lead (ii) compounds. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It is slightly soluble in cold water, but. in this video we will describe the equation pbcl2 + h2o and write what happens when. Lead Chloride Plus Water.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Lead Chloride Plus Water Because many lead (ii) compounds. It is slightly soluble in cold water, but. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead (ii) chloride is a white solid, melting at 501°c. Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. Unlike. Lead Chloride Plus Water.
From mungfali.com
Sodium Chloride Water Lead Chloride Plus Water in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is dissolved in water.when. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Unlike the tetrachlorides, lead(ii) chloride can be considered ionic in nature. lead (ii) chloride is a white solid,. Lead Chloride Plus Water.
From www.numerade.com
SOLVED Write balanced chemical equations, including states of matter Lead Chloride Plus Water lead (ii) chloride is a white solid, melting at 501°c. Because many lead (ii) compounds. It is very slightly soluble in cold water, but more soluble in hot water. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It is slightly soluble in cold water, but. Pbo 2 +. Lead Chloride Plus Water.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Plus Water Pbo 2 + 4 hcl → pbcl 2 + cl 2 + 2 h 2 o. It is slightly soluble in cold water, but. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. in this video we will describe the equation pbcl2 + h2o and write what happens when pbcl2 is. Lead Chloride Plus Water.