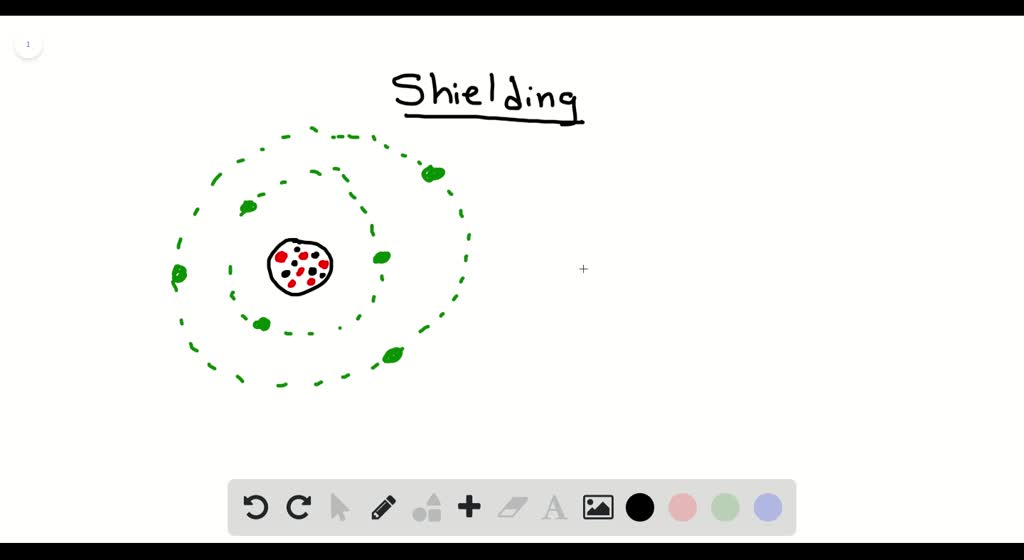
What Is Meant By Shielding Of Electrons In An Atom . shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy.
from ceyzfvbm.blob.core.windows.net
the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded.
V) What Is Meant By Shielding Of Electrons In An Atom at Christina
What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Hence, the nucleus has less grip on the outer electrons and are shielded. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons. What Is Meant By Shielding Of Electrons In An Atom.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The shielding effect describes the balance between the pull of the protons on valence electrons and the. What Is Meant By Shielding Of Electrons In An Atom.
From www.britannica.com
Periodic table Elements, Groups, Blocks Britannica What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which. What Is Meant By Shielding Of Electrons In An Atom.
From ar.inspiredpencil.com
Labeled Atom With Electron Cloud What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. The shielding effect describes. What Is Meant By Shielding Of Electrons In An Atom.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Hence, the nucleus has less grip on the outer electrons and are shielded. the concept of electron shielding,. What Is Meant By Shielding Of Electrons In An Atom.
From surfguppy.com
What is Electronegativity? What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are shielded. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding,. What Is Meant By Shielding Of Electrons In An Atom.
From www.lifeschemistrypress.com
5. The reality of electrons Life's Chemistry Press What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while. What Is Meant By Shielding Of Electrons In An Atom.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the. What Is Meant By Shielding Of Electrons In An Atom.
From www.numerade.com
SOLVED What is meant by the term "shielding of electrons" in an atom What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. What Is Meant By Shielding Of Electrons In An Atom.
From lillian-has-patton.blogspot.com
What Happens When an Electron Is Removed From an Atom LillianhasPatton What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive. What Is Meant By Shielding Of Electrons In An Atom.
From quiznutritions.z13.web.core.windows.net
How To Calculate Shielding Electrons What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. What Is Meant By Shielding Of Electrons In An Atom.
From spmkimia.onlinetuition.com.my
Susunan Elektron di Dalam Atom Nota Ulangkaji Kimia SPM Tingkatan 4 What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are. What Is Meant By Shielding Of Electrons In An Atom.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling. What Is Meant By Shielding Of Electrons In An Atom.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the. What Is Meant By Shielding Of Electrons In An Atom.
From www.chemistrysteps.com
NMR Chemical Shift ppm, Upfield, Downfield Chemistry Steps What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. What Is Meant By Shielding Of Electrons In An Atom.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus. What Is Meant By Shielding Of Electrons In An Atom.
From list.ly
School college chemistry A Listly List What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the. What Is Meant By Shielding Of Electrons In An Atom.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive. What Is Meant By Shielding Of Electrons In An Atom.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are shielded. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. shielding refers to the core. What Is Meant By Shielding Of Electrons In An Atom.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons. What Is Meant By Shielding Of Electrons In An Atom.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. What Is Meant By Shielding Of Electrons In An Atom.
From thechemistrynotes.com
Shielding effect What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while. What Is Meant By Shielding Of Electrons In An Atom.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know What Is Meant By Shielding Of Electrons In An Atom Hence, the nucleus has less grip on the outer electrons and are shielded. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. the concept of electron shielding, in which. What Is Meant By Shielding Of Electrons In An Atom.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. What Is Meant By Shielding Of Electrons In An Atom.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. shielding refers to the core electrons repelling the outer rings and thus lowering the. What Is Meant By Shielding Of Electrons In An Atom.
From enginelibrarydietrich.z19.web.core.windows.net
Label The Diagram Of An Atom What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which. What Is Meant By Shielding Of Electrons In An Atom.
From sciencenotes.org
List of Electron Configurations of Elements What Is Meant By Shielding Of Electrons In An Atom shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus. What Is Meant By Shielding Of Electrons In An Atom.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 What Is Meant By Shielding Of Electrons In An Atom the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. shielding refers to the core electrons repelling the outer rings and. What Is Meant By Shielding Of Electrons In An Atom.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive. What Is Meant By Shielding Of Electrons In An Atom.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the. What Is Meant By Shielding Of Electrons In An Atom.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding refers to the core electrons repelling the outer rings and thus lowering the. What Is Meant By Shielding Of Electrons In An Atom.