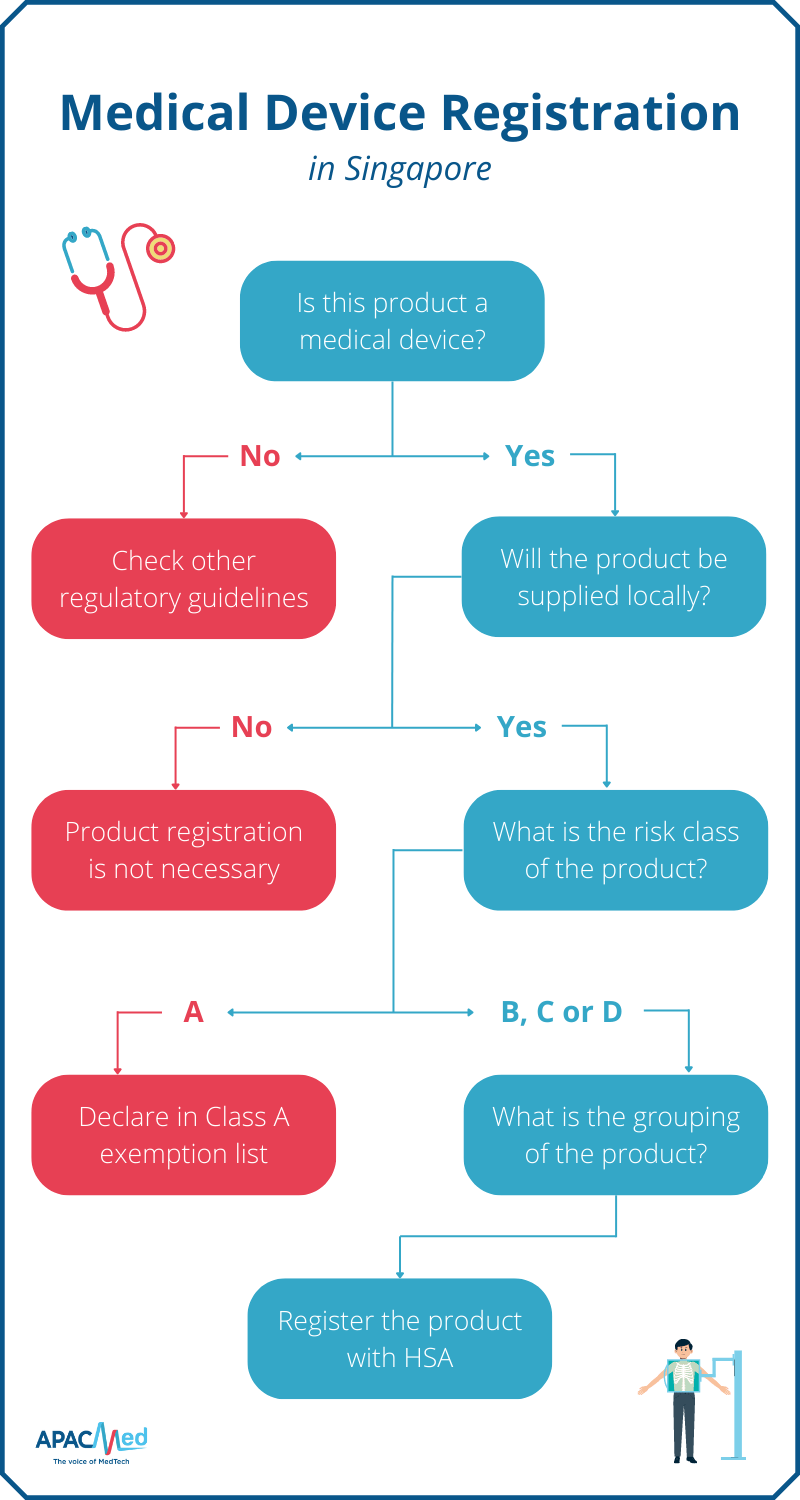
How Are Medical Devices Regulated . Regulation is based on rules about the development, validation, and maintenance of. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and welfare (mhlw). The purpose of regulation of medical devices: There are two regulatory authorities responsible for regulation of medical devices in japan:
from betebt.com
The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. There are two regulatory authorities responsible for regulation of medical devices in japan: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance of. The purpose of regulation of medical devices:
Medical Device Regulation Importance and Examples in APAC (2022)
How Are Medical Devices Regulated The purpose of regulation of medical devices: The purpose of regulation of medical devices: Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Regulation is based on rules about the development, validation, and maintenance of. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. There are two regulatory authorities responsible for regulation of medical devices in japan: The ministry of health, labour and welfare (mhlw). The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the.
From www.youtube.com
How Are Medical Devices Regulated? USA Canada UK EU YouTube How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. There are two regulatory authorities responsible for regulation of medical devices in japan: The medical device regulation (mdr), which. How Are Medical Devices Regulated.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies How Are Medical Devices Regulated The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The ministry of health, labour and welfare (mhlw). The purpose of regulation of medical devices: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. There are two regulatory authorities responsible for regulation of medical devices in. How Are Medical Devices Regulated.
From www.scribd.com
All Medical Devices in India To Be Regulated As "Drugs" Medical How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The medical device regulation. How Are Medical Devices Regulated.
From operonstrategist.com
GMP Certificate for Medical Devices (Standards and Requirements How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). There are two regulatory authorities responsible for regulation of medical devices in japan: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation is based on rules about the development, validation, and maintenance of. Medical devices are assigned to one of three regulatory. How Are Medical Devices Regulated.
From scientistssanctuary.com
eBOOK How to Achieve European Medical Device Registration and Apply a How Are Medical Devices Regulated The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation is based on rules about the development, validation, and maintenance of. The purpose of regulation of medical devices: Medical devices are assigned. How Are Medical Devices Regulated.
From machinery.directory
Medical Device Regulation Ensuring Safety and Efficacy How Are Medical Devices Regulated There are two regulatory authorities responsible for regulation of medical devices in japan: The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance of. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro. How Are Medical Devices Regulated.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. There. How Are Medical Devices Regulated.
From specculo.com
How are medical devices regulated in the EU? How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and welfare (mhlw). The purpose of regulation of medical devices: Regulation is based on rules about the development, validation, and maintenance of. Medical devices are assigned to one of three regulatory classes based on the level. How Are Medical Devices Regulated.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive How Are Medical Devices Regulated Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The ministry of health, labour and welfare (mhlw). There are two regulatory authorities responsible for regulation of medical devices in japan: The purpose of regulation of medical devices: Regulation is based on rules about the development, validation, and. How Are Medical Devices Regulated.
From www.youtube.com
7investing Explains How are Medical Devices and Tests How Are Medical Devices Regulated The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance of. Medical devices are assigned to one of. How Are Medical Devices Regulated.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). There are two regulatory authorities responsible for regulation of medical devices in japan: Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Regulation is based on rules about the development, validation, and maintenance of. The medical device regulation (mdr),. How Are Medical Devices Regulated.
From veraqueconsulting.com
Low risk and nonregulated medical devices Veraque Mexico How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance of. There are two. How Are Medical Devices Regulated.
From www.aqfmedical.com
Medical Devices Regulation How Are Medical Devices Regulated The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The purpose of regulation of medical devices: Regulation is based on rules about the development, validation, and maintenance of. The ministry of health,. How Are Medical Devices Regulated.
From info.orthocanada.com
How are medical devices regulated in Canada? How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The purpose of regulation of medical devices: Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The medicines and healthcare. How Are Medical Devices Regulated.
From www.eclevarmedtech.com
How are software medical devices currently regulated in Australia? How Are Medical Devices Regulated Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. There are two regulatory authorities responsible for regulation of medical devices in japan: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and. How Are Medical Devices Regulated.
From slideplayer.com
Addressing decade long of radiation safety in public How Are Medical Devices Regulated Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation is based on rules about the development, validation, and maintenance of. The ministry of health, labour and welfare (mhlw).. How Are Medical Devices Regulated.
From es.slideshare.net
Regulation of Medical Devices in US How Are Medical Devices Regulated The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. There are two regulatory authorities responsible for regulation of medical devices in japan: The ministry of health, labour and welfare (mhlw). The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The purpose of regulation of. How Are Medical Devices Regulated.
From gcpcentral.com
The Medical Device Regulation Why a Delay in Implementation, and What How Are Medical Devices Regulated Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance. How Are Medical Devices Regulated.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV How Are Medical Devices Regulated The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. There are two regulatory authorities responsible for regulation of medical devices in japan: The purpose of regulation of medical devices: Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and.. How Are Medical Devices Regulated.
From allevents.in
FDA Regulated Mobile Medical Apps as Devices and Cybersecurity How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. There are two regulatory authorities responsible for regulation of medical devices in japan: The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Regulation is based on rules about the development, validation, and maintenance of. The. How Are Medical Devices Regulated.
From www.eclevarmedtech.com
How are customized 3D printed medical devices currently regulated in How Are Medical Devices Regulated Regulation is based on rules about the development, validation, and maintenance of. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The purpose of regulation of medical devices: There are two regulatory. How Are Medical Devices Regulated.
From www.globalvoices.com
How Are Medical Devices Regulated In The EU? Global Voices How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. There are two regulatory authorities responsible for regulation of medical devices in japan: The purpose of regulation of medical. How Are Medical Devices Regulated.
From info.orthocanada.com
How are medical devices regulated in Canada? How Are Medical Devices Regulated Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The ministry of health, labour and welfare (mhlw). The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The purpose of regulation of medical devices: There are two regulatory authorities responsible for regulation. How Are Medical Devices Regulated.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) How Are Medical Devices Regulated There are two regulatory authorities responsible for regulation of medical devices in japan: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The ministry of health, labour and welfare (mhlw). The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Regulation is based on rules about. How Are Medical Devices Regulated.
From www.cleverdevsoftware.com
Wearable Devices in Healthcare Tech in Healthcare & Smart Medical How Are Medical Devices Regulated The purpose of regulation of medical devices: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The medical device regulation (mdr), which was adopted in april 2017, changes. How Are Medical Devices Regulated.
From www.linkedin.com
Freyr Medical Devices Regulatory Services on LinkedIn How are Near How Are Medical Devices Regulated There are two regulatory authorities responsible for regulation of medical devices in japan: The purpose of regulation of medical devices: The ministry of health, labour and welfare (mhlw). The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Regulation is based on rules about the development, validation, and maintenance of. The medical device regulation (mdr),. How Are Medical Devices Regulated.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems How Are Medical Devices Regulated The purpose of regulation of medical devices: Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. The ministry of health, labour and welfare (mhlw). The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices. How Are Medical Devices Regulated.
From www.tuvsud.com
Infographic The New Medical Device Regulation TÜV SÜD in India How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. There are two regulatory authorities responsible for regulation of medical devices in japan: Medical devices are assigned to one. How Are Medical Devices Regulated.
From www.linkedin.com
Medical Devices Compliances and Regulations in India How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The purpose of regulation of medical devices: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Regulation is based on rules about the development,. How Are Medical Devices Regulated.
From www.medicadepot.com
Why Should You Consider Using CEMarked Medical Devices? MEDICA DEPOT How Are Medical Devices Regulated The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and welfare (mhlw). The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. Regulation is based on rules about the development, validation, and maintenance of. Medical devices are assigned to one. How Are Medical Devices Regulated.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 How Are Medical Devices Regulated The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The ministry of health, labour and welfare (mhlw). Regulation is based on rules about the development, validation, and maintenance of. The purpose of regulation of medical devices: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed. How Are Medical Devices Regulated.
From specculo.com
How are medical devices regulated in the EU? How Are Medical Devices Regulated The ministry of health, labour and welfare (mhlw). The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. There are two regulatory authorities responsible for regulation of medical devices in japan: The purpose of regulation of medical devices: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746). How Are Medical Devices Regulated.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive How Are Medical Devices Regulated There are two regulatory authorities responsible for regulation of medical devices in japan: Regulation is based on rules about the development, validation, and maintenance of. The medicines and healthcare products regulatory agency (mhra) is responsible for regulating the uk medical. The purpose of regulation of medical devices: The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic. How Are Medical Devices Regulated.
From www.databridgemarketresearch.com
Healthcare Medical Devices Making it to Mainstream How Are Medical Devices Regulated The purpose of regulation of medical devices: The ministry of health, labour and welfare (mhlw). The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and. Regulation is based on. How Are Medical Devices Regulated.
From specculo.com
How are medical devices regulated in the EU? How Are Medical Devices Regulated The purpose of regulation of medical devices: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The regulations on medical devices (regulation (eu) 2017/745) and on in vitro diagnostic devices (regulation (eu) 2017/746) changed the. The ministry of health, labour and welfare (mhlw). There are two regulatory authorities responsible for regulation. How Are Medical Devices Regulated.