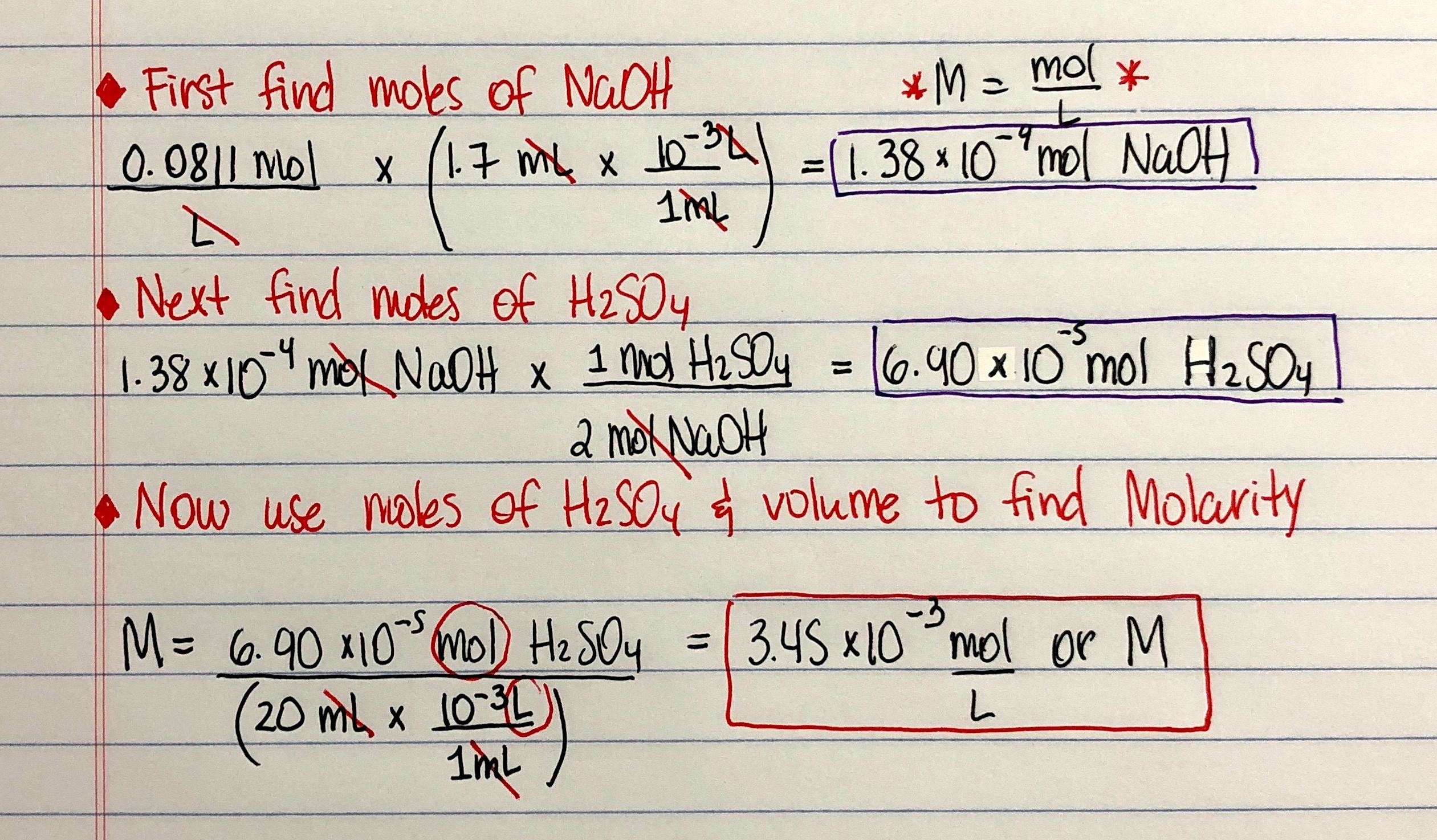Titration Practice Problems Answers . Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Titration calculations & answers 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Use the information to determine the concentration of the hydrochloric acid. Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. If ha is titrated with naoh, as soon as the first drop. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250.
from pdfprof.com
Find the requested quantities in the following problems: Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as the first drop. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use the information to determine the concentration of the hydrochloric acid. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration calculations & answers 1. What is the concentration of the hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution.
acid base titration problems with answers pdf
Titration Practice Problems Answers Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as the first drop. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration calculations & answers 1. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. What is the concentration of the hcl.
From pdfprof.com
acid base titration problems with answers pdf Titration Practice Problems Answers Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. • a. Titration Practice Problems Answers.
From www.studocu.com
Titration Curve Practice Problems MATH + SCIENCE INITIATIVE Titration Titration Practice Problems Answers If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use the information to determine the concentration of the hydrochloric acid. If ha is titrated with naoh, as soon as the first drop. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml. Titration Practice Problems Answers.
From lessoncampusspondyls.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Practice Problems Answers Use the information to determine the concentration of the hydrochloric acid. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize. Titration Practice Problems Answers.
From www.scribd.com
Titrations Practice Problems PDF Titration Practice Problems Answers If ha is titrated with naoh, as soon as the first drop. Titration calculations & answers 1. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. 1) it takes 83 ml of. Titration Practice Problems Answers.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Practice Problems Answers If ha is titrated with naoh, as soon as the first drop. Use the information to determine the concentration of the hydrochloric acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration calculations & answers 1. Find the requested quantities in the following problems: Use stoichiometry of the neutralization. Titration Practice Problems Answers.
From www.chem.fsu.edu
Quantitative Analysis Titration Practice Problems Answers If ha is titrated with naoh, as soon as the first drop. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems: Titration calculations. Titration Practice Problems Answers.
From www.studypool.com
SOLUTION Titrations questions with answers Studypool Titration Practice Problems Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. If ha is titrated with naoh, as soon as the first drop. Any titration involving a weak. Titration Practice Problems Answers.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Practice Problems Answers Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What is the concentration of the hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a. Titration Practice Problems Answers.
From www.studocu.com
Practice Problems Week 6 p H and Titrations With Answer Key Study Titration Practice Problems Answers Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. What is the concentration of the. Titration Practice Problems Answers.
From worksheets.clipart-library.com
SOLUTION 4chap dilutions and titration worksheet Studypool Titration Practice Problems Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If ha is titrated with naoh, as soon as the first drop. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Titration Practice Problems Answers.
From lessonmagicchampart.z14.web.core.windows.net
Titration Math Practice Problems Titration Practice Problems Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. What is the concentration. Titration Practice Problems Answers.
From www.youtube.com
Chapter 14 part 20, titration practice problem YouTube Titration Practice Problems Answers Titration calculations & answers 1. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Use the information to determine the concentration of the hydrochloric acid. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as. Titration Practice Problems Answers.
From www.studocu.com
Handout Ch 04, Titration Practice Problems Titration Practice Titration Practice Problems Answers Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If ha is titrated with naoh, as soon as the first drop. What is the concentration of the hcl. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Titration calculations & answers. Titration Practice Problems Answers.
From www.studocu.com
Test3 ch17b BufferTitrationEquilibrium Practice Problemsanswersfull Titration Practice Problems Answers What is the concentration of the hcl. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Find the requested quantities in the following problems: Titration calculations & answers 1. • a 25. Titration Practice Problems Answers.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Practice Problems Answers What is the concentration of the hcl. If ha is titrated with naoh, as soon as the first drop. Use the information to determine the concentration of the hydrochloric acid. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Titration calculations & answers 1. • a 25 cm3 sample of. Titration Practice Problems Answers.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Titration Practice Problems Answers If ha is titrated with naoh, as soon as the first drop. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water. Titration Practice Problems Answers.
From worksheets.clipart-library.com
Titration Practice Worksheet SCH 3uo PDF Sodium Hydroxide Titration Practice Problems Answers Titration calculations & answers 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in. Titration Practice Problems Answers.
From davida.davivienda.com
Titration Problems Worksheet Answers Printable Word Searches Titration Practice Problems Answers Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What is the concentration of the hcl. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution,. Titration Practice Problems Answers.
From www.chegg.com
Solved Practice Problems for AcidBase titration. Find the Titration Practice Problems Answers Use the information to determine the concentration of the hydrochloric acid. Find the requested quantities in the following problems: Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) it takes. Titration Practice Problems Answers.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Titration Practice Problems Answers Titration calculations & answers 1. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use the information to determine the concentration of the hydrochloric acid. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. 1) it takes 83 ml of a 0.45. Titration Practice Problems Answers.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Titration Practice Problems Answers Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. 1) it takes 83 ml. Titration Practice Problems Answers.
From www.chegg.com
Solved AcidBase Titration Practice Problems 1) Calculate Titration Practice Problems Answers Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. If ha. Titration Practice Problems Answers.
From www.tes.com
Titrations Home Learning Worksheet GCSE Teaching Resources Titration Practice Problems Answers Titration calculations & answers 1. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use the information to determine the concentration of the hydrochloric acid. Find the requested quantities in the following problems: What is the concentration of the hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved. Titration Practice Problems Answers.
From www.madebyteachers.com
Solution and Titration Stoichiometry a Chemistry Worksheet Made By Titration Practice Problems Answers Find the requested quantities in the following problems: • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Titration calculations & answers 1. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Any titration involving a weak a/b, from the first drop. Titration Practice Problems Answers.
From studylib.net
Titration Practice Worksheet Titration Practice Problems Answers Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the requested quantities in. Titration Practice Problems Answers.
From lessoncampusspondyls.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Practice Problems Answers • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use stoichiometry of the. Titration Practice Problems Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titrations Practice Worksheet Answers Titration Practice Problems Answers What is the concentration of the hcl. Find the requested quantities in the following problems: Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use stoichiometry of the neutralization to determine. Titration Practice Problems Answers.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Practice Problems Answers • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough. Titration Practice Problems Answers.
From www.youtube.com
Acidbase titration practice problems Stoichiometry YouTube Titration Practice Problems Answers • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What is the concentration of the hcl. Use the information to determine the concentration of the hydrochloric acid. Find the requested quantities in the following. Titration Practice Problems Answers.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Practice Problems Answers Titration calculations & answers 1. Any titration involving a weak a/b, from the first drop to the last, before equivalence, is a buffer problem. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. If ha is titrated with naoh, as soon as the first drop. If it takes 54 ml of. Titration Practice Problems Answers.
From classburns.z4.web.core.windows.net
Titration Practical Questions And Answers Titration Practice Problems Answers What is the concentration of the hcl. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Use the information to determine the concentration of the hydrochloric acid. Find the requested quantities in. Titration Practice Problems Answers.
From pdfprof.com
acid base titration problems with answers pdf Titration Practice Problems Answers Use the information to determine the concentration of the hydrochloric acid. If ha is titrated with naoh, as soon as the first drop. If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Find the requested quantities in the following problems: Titration calculations & answers 1. 1) it takes 83. Titration Practice Problems Answers.
From www.studocu.com
CHM 110 Chapter 10 Acids and BasesTitration Problems with answers Titration Practice Problems Answers Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Titration calculations & answers 1. Find the requested quantities in the following problems: If it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an hci solution, what is the. Any titration involving a weak a/b, from the first drop. Titration Practice Problems Answers.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Practice Problems Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems: What is the concentration of the hcl. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If ha is titrated with naoh, as. Titration Practice Problems Answers.
From www.scribd.com
IGCSE Titration Practice Questions Titration Practice Problems Answers Titration calculations & answers 1. • a 25 cm3 sample of hydrochloric acid is sucked into a pipette and transferred into a 250. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. A. Titration Practice Problems Answers.