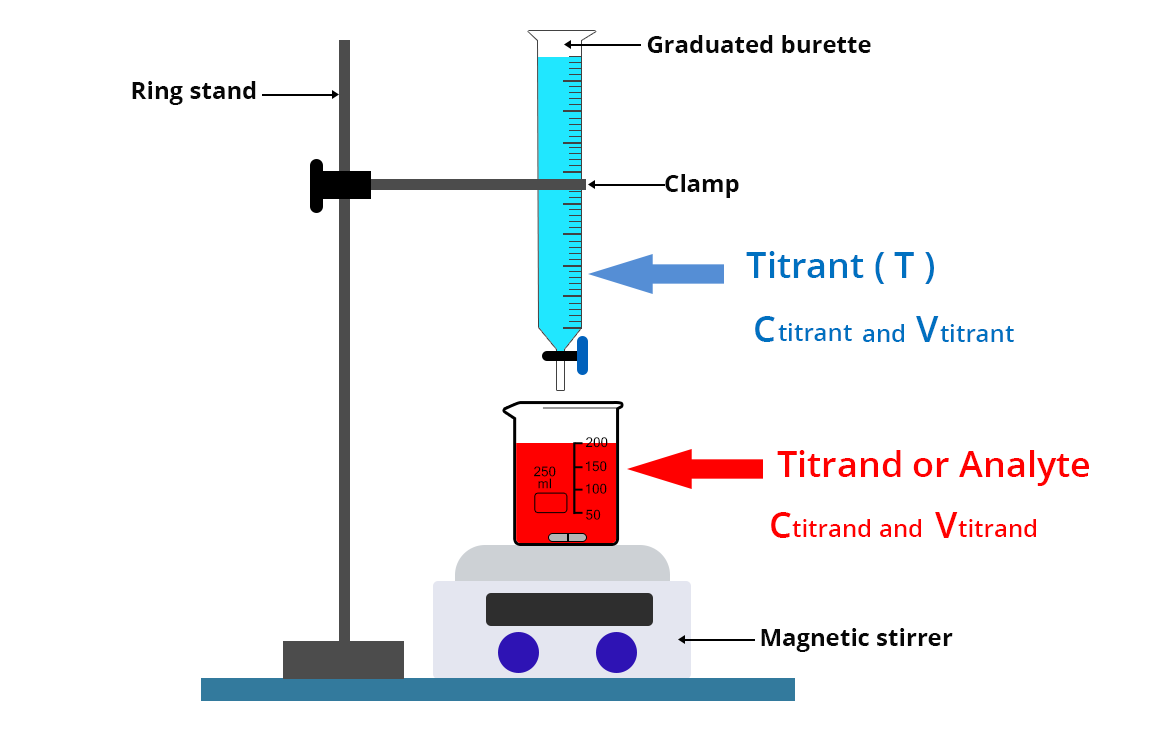
Burette Concentration In Titration . Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. The volume delivered by a burette can. Watch this demonstration of the correct titration procedure. Add solution from burette whilst swirling the mixture and add dropwise at end point. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. A burette is used when we are not sure of the exact volume of solution that will be required for reaction. To determine the concentration of one of the solutions in mol/dm. To determine the reacting volumes of solutions of an acid and alkali by titration. Distilled water can be added to the. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution.
from chimactiv.agroparistech.fr
A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. To determine the concentration of one of the solutions in mol/dm. A burette is used when we are not sure of the exact volume of solution that will be required for reaction. Watch this demonstration of the correct titration procedure. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. The volume delivered by a burette can. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. Distilled water can be added to the.
Chimactiv Interactive numerical educational resources for the
Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. The volume delivered by a burette can. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. To determine the concentration of one of the solutions in mol/dm. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. To determine the reacting volumes of solutions of an acid and alkali by titration. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. A burette is used when we are not sure of the exact volume of solution that will be required for reaction. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Add solution from burette whilst swirling the mixture and add dropwise at end point. Watch this demonstration of the correct titration procedure. Distilled water can be added to the.
From techblog.ctgclean.com
Chemistry What is Titration? CTG Clean Burette Concentration In Titration To determine the concentration of one of the solutions in mol/dm. Watch this demonstration of the correct titration procedure. Distilled water can be added to the. Add solution from burette whilst swirling the mixture and add dropwise at end point. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how. Burette Concentration In Titration.
From scientificservices.eu
Titration Burette UseScience Burette Concentration In Titration Watch this demonstration of the correct titration procedure. To determine the reacting volumes of solutions of an acid and alkali by titration. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. Distilled water can be added to the. Add solution from burette whilst. Burette Concentration In Titration.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog Burette Concentration In Titration A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. Distilled water can be added to the. A burette is used when we are. Burette Concentration In Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Burette Concentration In Titration Distilled water can be added to the. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. To determine the concentration of one of the solutions in mol/dm. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second. Burette Concentration In Titration.
From www.pathwaystochemistry.com
Titrations Introduction Pathways to Chemistry Burette Concentration In Titration Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. To determine the reacting volumes of solutions of an acid and alkali by titration. Add solution from burette whilst swirling the mixture and add dropwise at end point. Typically, the titrant (the known solution) is added from a. Burette Concentration In Titration.
From ck12.org
Titration CK12 Foundation Burette Concentration In Titration To determine the reacting volumes of solutions of an acid and alkali by titration. To determine the concentration of one of the solutions in mol/dm. Add solution from burette whilst swirling the mixture and add dropwise at end point. Watch this demonstration of the correct titration procedure. Typically, the titrant (the known solution) is added from a burette to a. Burette Concentration In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Burette Concentration In Titration Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. Watch this demonstration of the correct titration procedure. Distilled water can be added to the. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of. Burette Concentration In Titration.
From www.visionlearning.com
Ácidos y Bases I Chemistry Visionlearning Burette Concentration In Titration Distilled water can be added to the. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. To determine the concentration of one of the solutions in mol/dm. To determine the reacting volumes of solutions of an acid and alkali by titration. A burette. Burette Concentration In Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Burette Concentration In Titration To determine the concentration of one of the solutions in mol/dm. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. A burette (or “buret”) is a. Burette Concentration In Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. To determine the concentration of one of the solutions in mol/dm. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. To determine the reacting volumes of solutions. Burette Concentration In Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Burette Concentration In Titration The volume delivered by a burette can. To determine the reacting volumes of solutions of an acid and alkali by titration. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. Watch this demonstration of the correct titration procedure. A burette (or “buret”) is. Burette Concentration In Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Burette Concentration In Titration Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. Watch this demonstration of the correct titration procedure. To determine the concentration of one of the solutions in mol/dm. Distilled water can be added to the. Add solution from burette whilst swirling the mixture. Burette Concentration In Titration.
From acidsandbasesfordummieschem.weebly.com
Titration Acid and Bases for Dummies! Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. To determine the concentration of one of the solutions in mol/dm. Distilled water can be added to the. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second. Burette Concentration In Titration.
From www.dreamstime.com
Redox Titration with Burette and Pipette Stock Vector Illustration of Burette Concentration In Titration Add solution from burette whilst swirling the mixture and add dropwise at end point. Distilled water can be added to the. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. Watch this demonstration of the correct titration procedure. The volume delivered by a burette can. A burette. Burette Concentration In Titration.
From www.dreamstime.com
Titration stock photo. Image of biology, analyzing, burette 231791018 Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. To determine the concentration of one of the solutions in mol/dm. A burette is used when we are not sure of the exact volume of solution that will be required for reaction. Typically, the titrant (the known solution). Burette Concentration In Titration.
From www.shutterstock.com
Scientists Expelling Air Tip Burette Titration Stock Photo (Edit Now Burette Concentration In Titration A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a. Burette Concentration In Titration.
From researchersdays.science.lu
Wie funktioniert Titration? Burette Concentration In Titration A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. The volume delivered by a burette can. To determine the reacting volumes of solutions of an acid and. Burette Concentration In Titration.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Burette Concentration In Titration Watch this demonstration of the correct titration procedure. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. To determine the reacting volumes of solutions of an acid and alkali by titration. A burette is used to accurately measure the volume of liquid that has been allowed to. Burette Concentration In Titration.
From sbl1023.blogspot.com
LAB 3 TITRATION Burette Concentration In Titration To determine the concentration of one of the solutions in mol/dm. Distilled water can be added to the. Watch this demonstration of the correct titration procedure. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions. Burette Concentration In Titration.
From dxoftvpnv.blob.core.windows.net
Base Burette Function In Laboratory at Lucinda Jaimes blog Burette Concentration In Titration A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. To determine the concentration of one of the solutions in mol/dm. Watch this demonstration of the correct titration procedure. To determine the reacting volumes of solutions of an acid and alkali by titration. A burette is used when we. Burette Concentration In Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Burette Concentration In Titration Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. The volume delivered by a burette can. Add solution from burette whilst swirling the mixture and add dropwise at end point. Typically, the titrant (the known solution) is added from a burette to a known quantity of the. Burette Concentration In Titration.
From schempal.com
The Complete Guide to Understanding Burette Diagrams A StepbyStep Burette Concentration In Titration To determine the concentration of one of the solutions in mol/dm. To determine the reacting volumes of solutions of an acid and alkali by titration. Distilled water can be added to the. Add solution from burette whilst swirling the mixture and add dropwise at end point. Titration is a technique where a solution of a known concentration is used to. Burette Concentration In Titration.
From www.alamy.com
A burette used for titrations Stock Photo Alamy Burette Concentration In Titration Watch this demonstration of the correct titration procedure. Distilled water can be added to the. A burette is used when we are not sure of the exact volume of solution that will be required for reaction. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Add solution from. Burette Concentration In Titration.
From www.science-revision.co.uk
Titrations Burette Concentration In Titration A burette is used when we are not sure of the exact volume of solution that will be required for reaction. Add solution from burette whilst swirling the mixture and add dropwise at end point. The volume delivered by a burette can. Distilled water can be added to the. A buret is primarily used for titration to determine the concentration. Burette Concentration In Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Burette Concentration In Titration A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Distilled water can be added to the. Add solution from burette whilst swirling the mixture and add dropwise at end point. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for. Burette Concentration In Titration.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an Burette Concentration In Titration To determine the reacting volumes of solutions of an acid and alkali by titration. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Distilled water can be added to the. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte. Burette Concentration In Titration.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Burette Concentration In Titration Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. To determine the reacting volumes of solutions of an acid and alkali by titration. Add solution from burette whilst swirling the mixture and add dropwise at end point. A burette is used to accurately. Burette Concentration In Titration.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the Burette Concentration In Titration Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. A buret is primarily used for titration to determine the concentration of an unknown. Burette Concentration In Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. The volume delivered by a burette can. Watch this demonstration of the correct titration procedure. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the. Burette Concentration In Titration.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Burette Concentration In Titration A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much fluid. Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. A burette is used to accurately measure the volume of liquid that has been allowed to. Burette Concentration In Titration.
From www.brand.de
Titration with the bottletop burette Titrette® Burette Concentration In Titration A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. Distilled water can be added to the. A burette is used to accurately measure the volume of liquid that has been allowed to pour out of it. To determine the reacting volumes of solutions of an acid and alkali. Burette Concentration In Titration.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Burette Concentration In Titration Add solution from burette whilst swirling the mixture and add dropwise at end point. The volume delivered by a burette can. Typically, the titrant (the known solution) is added from a burette to a known quantity of the analyte (the second solution) until the reaction is complete. A buret is primarily used for titration to determine the concentration of an. Burette Concentration In Titration.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Burette Concentration In Titration Titration is a technique where a solution of a known concentration is used to determine the unknown concentration of a second solution. Watch this demonstration of the correct titration procedure. Add solution from burette whilst swirling the mixture and add dropwise at end point. Distilled water can be added to the. Typically, the titrant (the known solution) is added from. Burette Concentration In Titration.
From www.brand.de
Titration with the bottletop burette Titrette® Burette Concentration In Titration Distilled water can be added to the. A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. To determine the concentration of one of the solutions in mol/dm. A burette (or “buret”) is a handy lab tool for dispensing fluids into solutions and, more importantly, for measuring how much. Burette Concentration In Titration.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Burette Concentration In Titration A buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of known. The volume delivered by a burette can. Watch this demonstration of the correct titration procedure. Distilled water can be added to the. Add solution from burette whilst swirling the mixture and add dropwise at end point. A burette is. Burette Concentration In Titration.