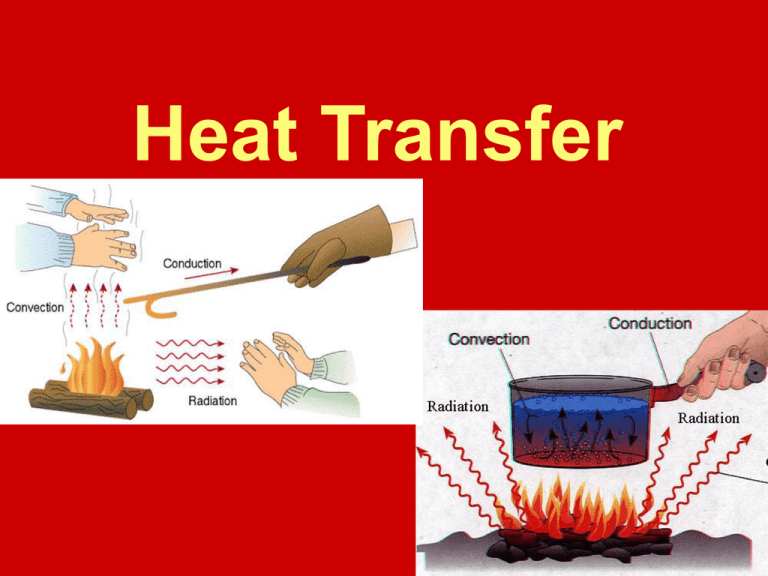
What Is Heat Transfer Melting . Explain some phenomena that involve conductive, convective, and radiative heat transfer; Heat from the air transfers to the ice causing it to melt. Melting of ice occurs in two steps: Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Heat is transferred from the soda to the ice for melting. To find the amount of ice melted,. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Solve problems on the relationships between heat. This amount of heat would raise the temperature of a gram of. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,.
from studylib.net
Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous. Heat is transferred from the soda to the ice for melting. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Heat from the air transfers to the ice causing it to melt. Solve problems on the relationships between heat. This amount of heat would raise the temperature of a gram of. To find the amount of ice melted,. Melting of ice occurs in two steps: The heat of fusion of ice, the heat required to melt one gram, is about 80 calories;
Heat Transfer
What Is Heat Transfer Melting Melting of ice occurs in two steps: This amount of heat would raise the temperature of a gram of. Solve problems on the relationships between heat. Explain some phenomena that involve conductive, convective, and radiative heat transfer; To find the amount of ice melted,. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Melting of ice occurs in two steps: This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat is transferred from the soda to the ice for melting. Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous. Heat from the air transfers to the ice causing it to melt. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories;
From www.studyiq.com
Heat Transfer Types, Definition, Convection, Radiation, Conduction What Is Heat Transfer Melting Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; This amount of heat would raise the temperature of a gram of. Heat from the air transfers to the ice causing. What Is Heat Transfer Melting.
From www.simscale.com
What Is Heat Transfer (Heat Flow)? Complete Guide SimScale What Is Heat Transfer Melting Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Explain some phenomena that involve conductive, convective, and radiative heat transfer; This amount of heat would raise the temperature of a gram. What Is Heat Transfer Melting.
From www.quia.com
Quia Heat Transfer Vocabulary Review What Is Heat Transfer Melting Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Heat is transferred from the soda to the ice for melting. Heat from the air transfers to the ice causing it to melt. Heat is a type of energy transfer that is caused by a temperature difference, and. What Is Heat Transfer Melting.
From www.chemicalslearning.com
Types of Convection Heat Transfer, Examples and Rate of Heat Transfer What Is Heat Transfer Melting First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. To find the amount of ice melted,. Explain. What Is Heat Transfer Melting.
From www.sciencefacts.net
Heat Transfer Definition, Types, And Examples What Is Heat Transfer Melting Solve problems on the relationships between heat. This amount of heat would raise the temperature of a gram of. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an. What Is Heat Transfer Melting.
From philschatz.com
Entropy and the Second Law of Thermodynamics Disorder and the What Is Heat Transfer Melting Explain some phenomena that involve conductive, convective, and radiative heat transfer; This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. To find the amount of ice melted,. Heat from the air transfers to the ice causing it to melt. The heat of fusion of ice, the heat required to melt. What Is Heat Transfer Melting.
From eduinput.com
10 Examples of Heat Transfer What Is Heat Transfer Melting This amount of heat would raise the temperature of a gram of. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Melting of ice occurs in two steps:. What Is Heat Transfer Melting.
From www.vectorstock.com
Diagram showing how heat transfer Royalty Free Vector Image What Is Heat Transfer Melting Explain some phenomena that involve conductive, convective, and radiative heat transfer; This amount of heat would raise the temperature of a gram of. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous.. What Is Heat Transfer Melting.
From fr.slideshare.net
Chapter 4 igneous rocks What Is Heat Transfer Melting Solve problems on the relationships between heat. Melting of ice occurs in two steps: Heat from the air transfers to the ice causing it to melt. Heat is transferred from the soda to the ice for melting. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. This. What Is Heat Transfer Melting.
From slideplayer.com
Heat Transfer. ppt download What Is Heat Transfer Melting The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous. Solve problems on the relationships between heat. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Melting of ice occurs. What Is Heat Transfer Melting.
From studylib.net
Heat Transfer What Is Heat Transfer Melting Heat is transferred from the soda to the ice for melting. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. This amount of heat would raise the temperature of a gram of. The heat of fusion of ice, the heat required to melt one gram, is about. What Is Heat Transfer Melting.
From imnooriii.blogspot.com
HeatFacts about the heat. Heat transfer. Why does heat transfer from What Is Heat Transfer Melting To find the amount of ice melted,. Heat is transferred from the soda to the ice for melting. This amount of heat would raise the temperature of a gram of. Solve problems on the relationships between heat. Melting of ice occurs in two steps: Explain some phenomena that involve conductive, convective, and radiative heat transfer; Heat from the air transfers. What Is Heat Transfer Melting.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation What Is Heat Transfer Melting Melting of ice occurs in two steps: This amount of heat would raise the temperature of a gram of. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Heat is transferred from the soda to the ice for melting. Heat is a type of energy transfer that. What Is Heat Transfer Melting.
From joijbodgi.blob.core.windows.net
Heat Transfer Through Convection Examples at Amy Campbell blog What Is Heat Transfer Melting Heat from the air transfers to the ice causing it to melt. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Explain some phenomena. What Is Heat Transfer Melting.
From sciencenotes.org
Heat Transfer Conduction, Convection, Radiation What Is Heat Transfer Melting The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Explain some phenomena that involve conductive, convective, and radiative heat transfer; This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat from the air transfers to the ice causing it to melt. Heat. What Is Heat Transfer Melting.
From www.studypool.com
SOLUTION Conduction and convection heat transfer chapter 1 What Is Heat Transfer Melting To find the amount of ice melted,. Melting of ice occurs in two steps: Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. First. What Is Heat Transfer Melting.
From slideplayer.com
Heat Transfer. ppt download What Is Heat Transfer Melting Solve problems on the relationships between heat. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Heat from the air transfers to the ice causing it to melt. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. To find the amount of ice melted,. Heat transfer processes set. What Is Heat Transfer Melting.
From studiousguy.com
13 Examples Of Convection In Everyday Life StudiousGuy What Is Heat Transfer Melting Heat is transferred from the soda to the ice for melting. This amount of heat would raise the temperature of a gram of. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Heat from the air. What Is Heat Transfer Melting.
From www.slideshare.net
Thermodynamics and Heat Transfer What Is Heat Transfer Melting Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Heat from the air transfers to the ice causing it to melt. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Solve problems on the relationships between heat. Melting of ice occurs in two steps: First. What Is Heat Transfer Melting.
From www.slideserve.com
PPT Momentum Heat Mass Transfer PowerPoint Presentation ID1470834 What Is Heat Transfer Melting Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Heat transfer processes set limits to the performance of aerospace components and systems and the subject is one of an enormous. Energy is required to melt. What Is Heat Transfer Melting.
From www.nuclear-power.com
Heat Transfer Definition, Mechanisms & Application What Is Heat Transfer Melting Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Melting of ice occurs in two steps: Solve problems on the relationships between heat. Heat from the air transfers to the ice causing it to melt.. What Is Heat Transfer Melting.
From mungfali.com
Heat Transfer Melting What Is Heat Transfer Melting First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Solve problems on the relationships between heat. Melting of ice occurs in two steps: This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat is transferred from the soda to the ice for melting.. What Is Heat Transfer Melting.
From www.mdpi.com
Materials Free FullText Heat Source Modeling in Selective Laser What Is Heat Transfer Melting To find the amount of ice melted,. Solve problems on the relationships between heat. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. This amount of heat would raise the temperature of a gram of. Heat transfer processes set limits to the performance of aerospace components and systems and the. What Is Heat Transfer Melting.
From www.britannica.com
Heat transfer Definition & Facts Britannica What Is Heat Transfer Melting Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. To find the amount of ice melted,. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Heat is transferred from the soda to the ice for melting. Explain some phenomena that. What Is Heat Transfer Melting.
From mepacademy.com
How Heat Transfer Works MEP Academy What Is Heat Transfer Melting First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Heat from the air transfers to the ice causing it to melt. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Energy is required to. What Is Heat Transfer Melting.
From www.slideserve.com
PPT Volcanic Hazards PowerPoint Presentation, free download ID1954493 What Is Heat Transfer Melting The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Solve problems on the relationships between heat. This amount of heat would raise the temperature of a gram of. Explain some phenomena that involve conductive, convective, and radiative heat transfer; This question involves both heat for a phase change (melting of ice) and. What Is Heat Transfer Melting.
From studylib.net
Melting Ice Cubes aka. Thermodynamics and Heat Transfer What Is Heat Transfer Melting Melting of ice occurs in two steps: The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. Heat from the air transfers to the ice causing it to melt. Solve problems on the relationships between heat. Heat. What Is Heat Transfer Melting.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Is Heat Transfer Melting Heat is transferred from the soda to the ice for melting. To find the amount of ice melted,. The heat of fusion of ice, the heat required to melt one gram, is about 80 calories; Melting of ice occurs in two steps: Heat is a type of energy transfer that is caused by a temperature difference, and it can change. What Is Heat Transfer Melting.
From www.sciencelearn.org.nz
Material World Melting — Science Learning Hub What Is Heat Transfer Melting To find the amount of ice melted,. Melting of ice occurs in two steps: Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. This. What Is Heat Transfer Melting.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic What Is Heat Transfer Melting First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. To find the amount of ice melted,. Explain some phenomena that involve conductive, convective, and radiative heat transfer; Melting of ice occurs in two steps: This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction.. What Is Heat Transfer Melting.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Is Heat Transfer Melting Heat from the air transfers to the ice causing it to melt. This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. This amount of heat would raise. What Is Heat Transfer Melting.
From pressbooks.bccampus.ca
7.1 Magma and How It Forms Physical Geology H5P Edition V1.1 What Is Heat Transfer Melting Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so. The heat of fusion of ice, the heat required to melt one gram, is about. What Is Heat Transfer Melting.
From geologylearn.blogspot.pt
Why does magma form? Learning Geology What Is Heat Transfer Melting Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. Melting of ice occurs in two steps: First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. The heat of fusion of ice, the heat required to melt one gram, is. What Is Heat Transfer Melting.
From eduinput.com
What is Heat Transfer?Definition, And Types What Is Heat Transfer Melting Heat from the air transfers to the ice causing it to melt. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. This amount of heat would raise the temperature of a gram of. Solve problems on the relationships between heat. Energy is required to melt a solid because the cohesive bonds between the. What Is Heat Transfer Melting.
From www.slideserve.com
PPT Momentum Heat Mass Transfer PowerPoint Presentation, free What Is Heat Transfer Melting This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature,. This amount. What Is Heat Transfer Melting.