Fda Expiration Date Requirements Medical Devices . Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. How are expiration dates established? Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf.
from pharmaknowl.com
Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. How are expiration dates established? Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be.
SFDA Labelling Requirements PharmaKnowl
Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Fda regulations require drug applicants to provide stability testing data with a proposed expiration.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. How. Fda Expiration Date Requirements Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed. Fda Expiration Date Requirements Medical Devices.
From studylib.net
Good Practice Guidance 4 Expiry Dates for Medication Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Whenever the label of a medical device includes a printed expiration date, date of manufacture,. Fda Expiration Date Requirements Medical Devices.
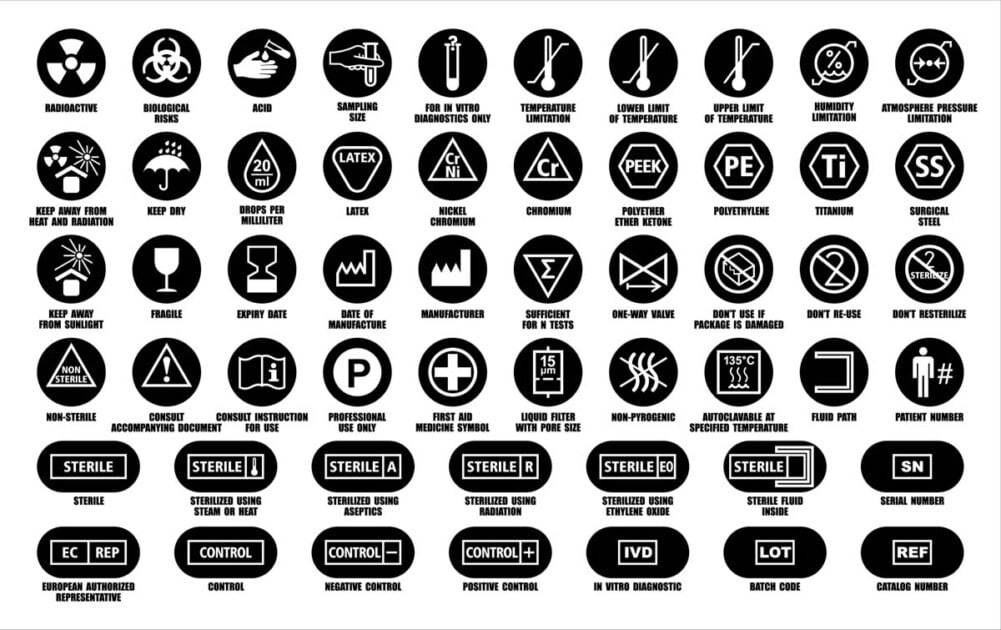
From mungfali.com
FDA Medical Device Label Symbols Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes a printed expiration. Fda Expiration Date Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Whenever the label of a medical device includes. Fda Expiration Date Requirements Medical Devices.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Fda regulations require drug applicants to provide. Fda Expiration Date Requirements Medical Devices.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Fda Expiration Date Requirements Medical Devices.
From knowleswellness.com
Medication Expiration Date Guidelines by FDA KnowlesWellness Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. How are expiration dates established? (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Inform readers of the food and drug administration (fda) regulations and policies relating. Fda Expiration Date Requirements Medical Devices.
From stock.adobe.com
Instruction of FDA number, manufacturing date, and expiry date label of Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended. Fda Expiration Date Requirements Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical device includes a printed. Fda Expiration Date Requirements Medical Devices.
From knowleswellness.com
Medication Expiration Date Guidelines by FDA KnowlesWellness Fda Expiration Date Requirements Medical Devices How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date. Fda Expiration Date Requirements Medical Devices.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Whenever the label of a medical device includes a printed expiration date, date of. Fda Expiration Date Requirements Medical Devices.
From fra.animalia-life.club
Iso 15223 1 Symboles Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Fda regulations require. Fda Expiration Date Requirements Medical Devices.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. In the table below, the expiration date column lists where to find the. Fda Expiration Date Requirements Medical Devices.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. How are expiration dates established? (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes. Fda Expiration Date Requirements Medical Devices.
From knowleswellness.com
Medication Expiration Date Guidelines by FDA KnowlesWellness Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a. Fda Expiration Date Requirements Medical Devices.
From www.scribd.com
FDA Requirements for Medical Devices Medical Device Authentication Fda Expiration Date Requirements Medical Devices In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Whenever the label of a medical device. Fda Expiration Date Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Expiration Date Requirements Medical Devices In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Inform readers of the food and drug administration. Fda Expiration Date Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Expiration Date Requirements Medical Devices (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration. Fda Expiration Date Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. How are expiration dates established? In the table below, the expiration date column lists where to find the expiration date for that test, and. Fda Expiration Date Requirements Medical Devices.
From www.linkedin.com
FDA Medical Device Requirements & Regulations. Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. In the table below, the. Fda Expiration Date Requirements Medical Devices.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Whenever the label of a medical device includes. Fda Expiration Date Requirements Medical Devices.
From www.slideserve.com
PPT FDA regulatory requirements for medical devices PowerPoint Fda Expiration Date Requirements Medical Devices How are expiration dates established? In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user. Fda Expiration Date Requirements Medical Devices.
From www.presentationeze.com
FDA Regulatory Requirements Medical Devices.PresentationEZE Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Whenever the label of a medical device. Fda Expiration Date Requirements Medical Devices.
From www.presentationeze.com
FDA Validation Requirements for Medical Devices PresentationEZE Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. How are expiration dates established? Whenever the. Fda Expiration Date Requirements Medical Devices.
From www.presentationeze.com
Medical Device Regulations. Design Requirements PresentationEZE Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended. Fda Expiration Date Requirements Medical Devices.
From ger.animalia-life.club
ISO 15223 1 2023 Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. (a) to assure that a. Fda Expiration Date Requirements Medical Devices.
From knowleswellness.com
Medication Expiration Date Guidelines by FDA KnowlesWellness Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to. Fda Expiration Date Requirements Medical Devices.
From medicaldeviceacademy.com
How to find updated FDA forms for a 510k Medical Device Academy Fda Expiration Date Requirements Medical Devices Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a. Fda Expiration Date Requirements Medical Devices.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Fda Expiration Date Requirements Medical Devices Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended. Fda Expiration Date Requirements Medical Devices.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to. Fda Expiration Date Requirements Medical Devices.
From www.healthcarepackaging.com
FDA Guidance Expiration Dating of UnitDose Repackaged Solid Oral Fda Expiration Date Requirements Medical Devices (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended. Fda Expiration Date Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Expiration Date Requirements Medical Devices In the table below, the expiration date column lists where to find the expiration date for that test, and the other details column lists the shelf. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended. Fda Expiration Date Requirements Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Expiration Date Requirements Medical Devices Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be. Inform readers of the food and drug administration (fda) regulations and policies relating to shelf life of medical devices. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date. Fda Expiration Date Requirements Medical Devices.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Fda Expiration Date Requirements Medical Devices (a) to assure that a drug product meets applicable standards of identity, strength, quality, and purity. Fda regulations require drug applicants to provide stability testing data with a proposed expiration. How are expiration dates established? Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Fda Expiration Date Requirements Medical Devices.