Standard Curve And Beer's Law . The second step of the process is to generate a standard curve. The law states that a chemical's concentration is directly proportional to a solution's. It is a graph that shows the relationship between different known concentrations of a. Beer’s law uses a maths approach to explain the experimentally obtained. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is an equation that relates light's attenuation to a material's properties. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law explains the relationship between cordial concentration and light.
from stock.adobe.com
Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law uses a maths approach to explain the experimentally obtained. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. The second step of the process is to generate a standard curve. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It is a graph that shows the relationship between different known concentrations of a. The law states that a chemical's concentration is directly proportional to a solution's. Beer's law explains the relationship between cordial concentration and light.
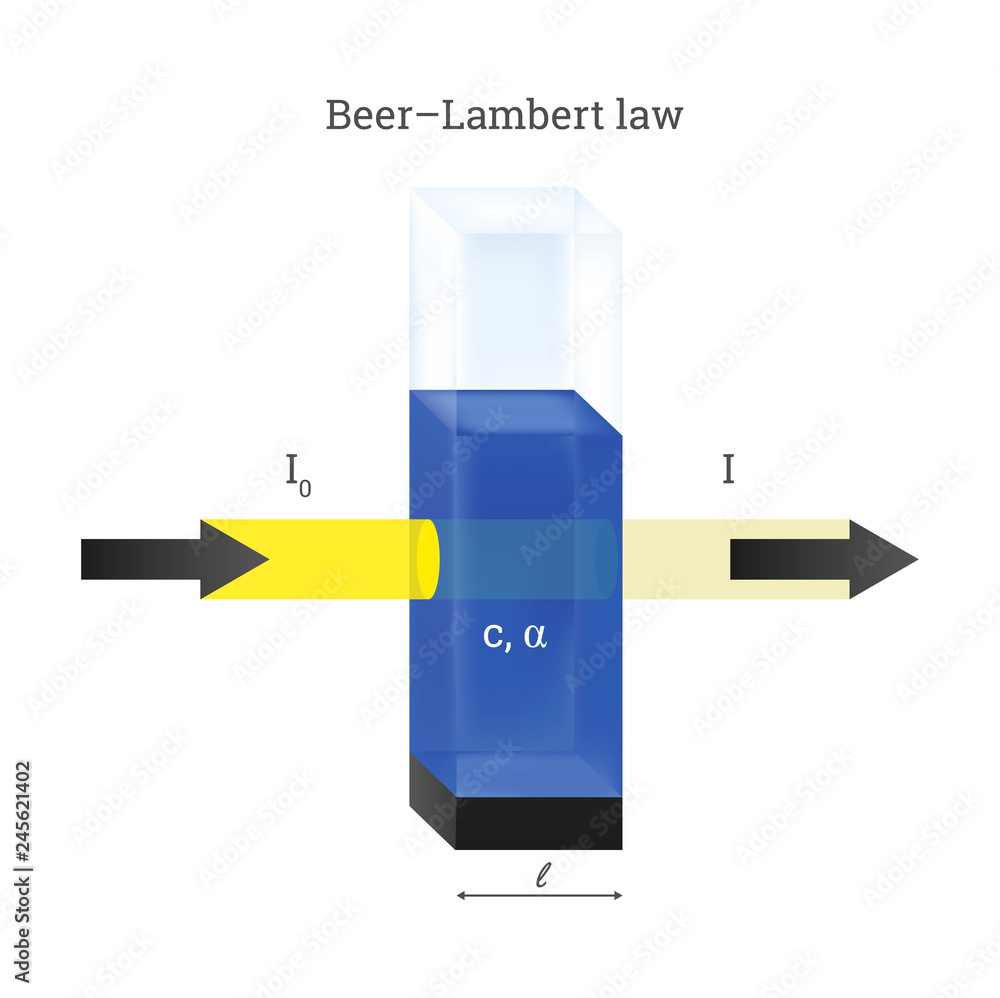
Vector scheme of Beer Lambert law. Cuvette with the blue liquid sample
Standard Curve And Beer's Law Beer’s law uses a maths approach to explain the experimentally obtained. Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that shows the relationship between different known concentrations of a. The second step of the process is to generate a standard curve. The law states that a chemical's concentration is directly proportional to a solution's. Beer's law explains the relationship between cordial concentration and light. Beer’s law uses a maths approach to explain the experimentally obtained. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
From www.thoughtco.com
Beer's Law Definition and Equation Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The second step of the process is to generate a standard curve. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is an equation that relates light's attenuation to a material's properties. It is a. Standard Curve And Beer's Law.
From www.chegg.com
Solved c. PREPARATION OF STANDARD CURVE AND BEER'S LAW Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law explains the relationship between cordial concentration and light. It is a graph that shows the relationship between different known concentrations of a. The second step of the process is to generate a standard curve. Beer’s law uses a maths. Standard Curve And Beer's Law.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law is an equation that relates light's attenuation to a material's properties. The second step of the process is to generate a standard curve. Beer’s law uses a maths approach. Standard Curve And Beer's Law.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Standard Curve And Beer's Law The second step of the process is to generate a standard curve. Beer’s law uses a maths approach to explain the experimentally obtained. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that. Standard Curve And Beer's Law.
From www.purechemistry.org
BeerLambert Law Purechemistry Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The law states that a chemical's concentration is directly. Standard Curve And Beer's Law.
From www.researchgate.net
Fig. S2. Beer's law calibration curve for the UVVis spectrum of Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. The law states that a chemical's concentration is directly proportional to a solution's. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s. Standard Curve And Beer's Law.
From www.chegg.com
Table A Generation of Standard/Calibration Curve Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The law states that a chemical's concentration is directly proportional to a solution's. The second step of the process. Standard Curve And Beer's Law.
From stock.adobe.com
Vector scheme of Beer Lambert law. Cuvette with the blue liquid sample Standard Curve And Beer's Law The second step of the process is to generate a standard curve. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that shows the relationship between different known concentrations of a. The law. Standard Curve And Beer's Law.
From www.chegg.com
Solved Examine the given Beer's law standard curve for an Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The second step of the process is to generate a standard curve. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. It is a graph that shows the relationship between different known concentrations of a. Beer’s law uses a maths. Standard Curve And Beer's Law.
From plot.ly
Beer's Law Plot scatter chart made by Java789 plotly Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. Beer’s law uses a maths approach to explain the experimentally obtained. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law explains the relationship between cordial concentration and light. The amount of light that a. Standard Curve And Beer's Law.
From www.chegg.com
Solved Examine the given Beer's law standard curve for an Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. The law states that a chemical's concentration is directly proportional to a solution's. The second step of the process is to generate a standard curve. Beer’s law. Standard Curve And Beer's Law.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Standard Curve And Beer's Law The law states that a chemical's concentration is directly proportional to a solution's. The second step of the process is to generate a standard curve. Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that shows the relationship between different known concentrations of a. Equation 8.2.3 and equation 8.2.4, which establish the. Standard Curve And Beer's Law.
From www.researchgate.net
Fig. S1. Beer's law calibration curve for the UVVis spectrum of Standard Curve And Beer's Law The second step of the process is to generate a standard curve. The law states that a chemical's concentration is directly proportional to a solution's. Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It is a. Standard Curve And Beer's Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. The second step of the process is to generate a standard curve. Beer's law explains the relationship between cordial concentration and light. It is a graph that shows the relationship between different known concentrations of a. The law states that a. Standard Curve And Beer's Law.
From www.chegg.com
Solved A) Standard solution and Beer's law calibration curve Standard Curve And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law explains the relationship between cordial concentration and light. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer’s law uses a maths approach to explain the. Standard Curve And Beer's Law.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Standard Curve And Beer's Law The second step of the process is to generate a standard curve. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law uses a maths approach to explain the experimentally obtained. The amount of light that. Standard Curve And Beer's Law.
From www.researchgate.net
Beer's law curves for method A, method B and method C Download Standard Curve And Beer's Law Beer’s law uses a maths approach to explain the experimentally obtained. The law states that a chemical's concentration is directly proportional to a solution's. Beer's law explains the relationship between cordial concentration and light. The second step of the process is to generate a standard curve. Beer's law is an equation that relates light's attenuation to a material's properties. Equation. Standard Curve And Beer's Law.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Standard Curve And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is an equation that relates light's attenuation to a material's properties. The second step of the process is to generate a standard curve. Beer's law explains the relationship between cordial concentration and light. Equation 8.2.3 and. Standard Curve And Beer's Law.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. It is a graph that shows the relationship between different known concentrations of a. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's. The. Standard Curve And Beer's Law.
From www.youtube.com
Standard Curve & Error Bars ⇔ [c] 💻 Beer's Law Linear Regression (3 Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. The second step of the process is to generate a standard curve. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's. Beer’s law uses. Standard Curve And Beer's Law.
From www.researchgate.net
Fig. S2. Beer's law calibration curve for the UVVis spectrum of Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is an equation that relates light's attenuation to a material's properties. Beer's law explains the relationship between cordial concentration and light. Equation. Standard Curve And Beer's Law.
From klawmisjt.blob.core.windows.net
Beer's Law Plot Excel at Harold Osborne blog Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. The law states that a chemical's concentration is directly proportional to a solution's. Beer’s law uses a maths approach to explain the experimentally obtained. Beer's law is an equation that relates light's attenuation to a material's properties. Equation 8.2.3 and equation 8.2.4, which establish the linear. Standard Curve And Beer's Law.
From www.chegg.com
Solved A) Standard solution and Beer's law calibration curve Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's. It is. Standard Curve And Beer's Law.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The second step of the process is to generate a standard curve. The law states that a chemical's concentration is directly proportional to a solution's. Beer’s law uses. Standard Curve And Beer's Law.
From www.chegg.com
Solved A) Standard solution and Beer's law calibration curve Standard Curve And Beer's Law The second step of the process is to generate a standard curve. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law uses a maths approach to explain the experimentally obtained. Beer's law is an equation that relates light's attenuation to a material's properties. It is. Standard Curve And Beer's Law.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation ID2407366 Standard Curve And Beer's Law Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. It is a graph that shows the relationship between different known concentrations of a. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The second step of the. Standard Curve And Beer's Law.
From www.researchgate.net
Fig. S3. Beer's law calibration curve for the UVVis spectrum of Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The law states that a chemical's concentration is directly proportional to a solution's. The second step of the process is to generate a standard curve. Beer’s law uses. Standard Curve And Beer's Law.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Standard Curve And Beer's Law Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that shows the relationship between different known concentrations of a. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The second step of the process is to generate a standard. Standard Curve And Beer's Law.
From www.scientistcindy.com
Chemistry and Conversions Lab SCIENTIST CINDY Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. Beer’s law uses a maths approach to explain the experimentally obtained. The second step of the process is to generate a standard curve. Beer's law is an equation that relates light's attenuation to a material's properties. It is a graph that shows the relationship between different known concentrations of a.. Standard Curve And Beer's Law.
From www.chegg.com
Solved Examine the given Beer's law standard curve for an Standard Curve And Beer's Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It is a graph that shows the relationship between different known concentrations of a. The second step of the process is to generate a standard curve. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance. Standard Curve And Beer's Law.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Standard Curve And Beer's Law The law states that a chemical's concentration is directly proportional to a solution's. Beer's law explains the relationship between cordial concentration and light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law uses a maths approach to explain the experimentally obtained. It is a graph. Standard Curve And Beer's Law.
From www.researchgate.net
Fig. S3. Beer's law calibration curve for the UVVis spectrum of Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. The second step of the process is to generate a standard curve. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's. Beer’s law uses a maths approach to explain the experimentally obtained. It. Standard Curve And Beer's Law.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. Beer's law explains the relationship between cordial concentration and light. The second step of the process is to generate a standard curve. Equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer’s law uses a maths. Standard Curve And Beer's Law.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Standard Curve And Beer's Law It is a graph that shows the relationship between different known concentrations of a. Beer's law explains the relationship between cordial concentration and light. Beer's law is an equation that relates light's attenuation to a material's properties. The second step of the process is to generate a standard curve. The law states that a chemical's concentration is directly proportional to. Standard Curve And Beer's Law.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Standard Curve And Beer's Law Beer's law explains the relationship between cordial concentration and light. Beer’s law uses a maths approach to explain the experimentally obtained. The law states that a chemical's concentration is directly proportional to a solution's. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Equation 8.2.3 and equation. Standard Curve And Beer's Law.