Pressure Equation Chem . The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. These mean exactly the same thing. Identify the mathematical relationships between the various properties of. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Learn and apply boyle’s law. By the end of this section, you will be able to: Learn what is meant by the term gas laws. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Pressure is force per unit area of surface; The value of the ideal gas constant, r, depends on the other units chosen for the formula.
from general.chemistrysteps.com
Learn and apply boyle’s law. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. By the end of this section, you will be able to: Learn what is meant by the term gas laws. The value of the ideal gas constant, r, depends on the other units chosen for the formula. These mean exactly the same thing. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Pressure is force per unit area of surface;
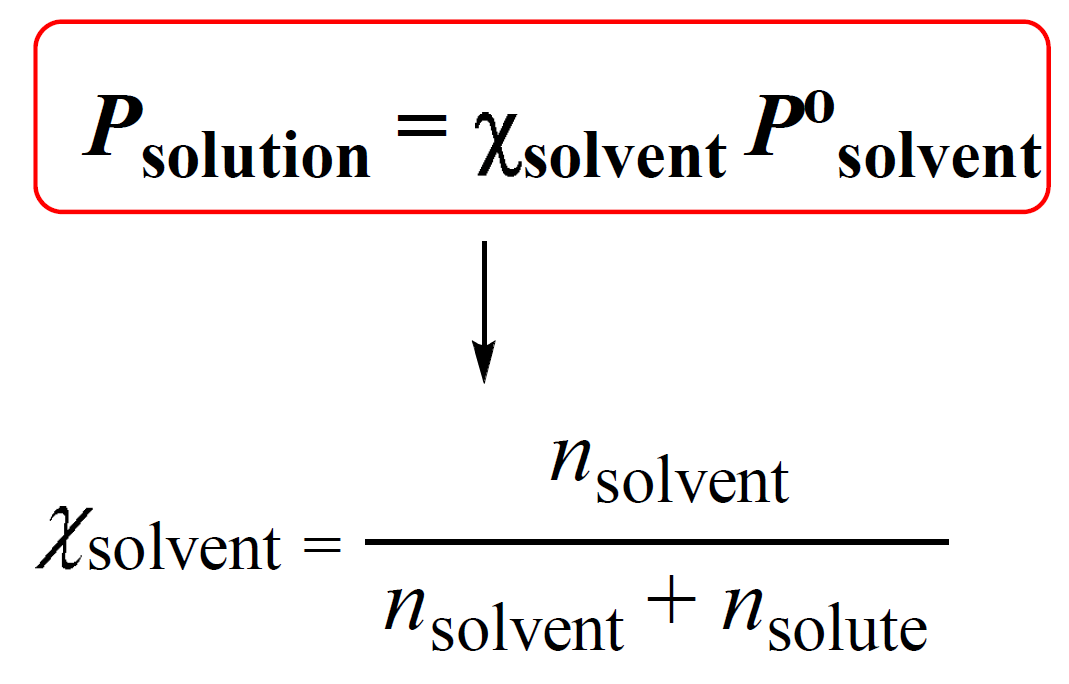
Vapor Pressure Lowering Chemistry Steps
Pressure Equation Chem The value of the ideal gas constant, r, depends on the other units chosen for the formula. These mean exactly the same thing. Learn and apply boyle’s law. Identify the mathematical relationships between the various properties of. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The value of the ideal gas constant, r, depends on the other units chosen for the formula. By the end of this section, you will be able to: Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Learn what is meant by the term gas laws. Pressure is force per unit area of surface;
From sciencenotes.org
Clausius Clapeyron Equation Pressure Equation Chem These mean exactly the same thing. Pressure is force per unit area of surface; The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). By the end of this section, you will be able to: Identify the mathematical relationships between the various properties of. Ideal gas law, relation between the pressure p,. Pressure Equation Chem.
From www.youtube.com
Pressure Formula. What Is The Formula For Working Out Pressure? YouTube Pressure Equation Chem The value of the ideal gas constant, r, depends on the other units chosen for the formula. Pressure is force per unit area of surface; Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Identify the mathematical relationships between. Pressure Equation Chem.
From www.wikipremed.com
The WikiPremed MCAT Course Image Archive Formula for pressure Pressure Equation Chem Learn what is meant by the term gas laws. Learn and apply boyle’s law. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Pressure is force per unit area of surface;. Pressure Equation Chem.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor Pressure Equation Chem Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. These mean exactly the same thing. Pressure is force per unit area of surface; Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas. Pressure Equation Chem.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Pressure Equation Chem These mean exactly the same thing. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. The value of the ideal gas constant, r, depends on the other units chosen for the formula. Identify the mathematical relationships between the various properties of. The si unit for pressure is. Pressure Equation Chem.
From slidesharenow.blogspot.com
Pressure And Volume Relationship Formula slideshare Pressure Equation Chem These mean exactly the same thing. By the end of this section, you will be able to: Identify the mathematical relationships between the various properties of. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding.. Pressure Equation Chem.
From www.youtube.com
Coefficient of Pressure Formula YouTube Pressure Equation Chem Pressure is force per unit area of surface; Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The value of the ideal gas constant, r, depends on the other units chosen. Pressure Equation Chem.
From www.vrogue.co
Osmotic Pressure Chemistry Steps vrogue.co Pressure Equation Chem Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. These mean exactly the same thing. Learn what is meant by the term gas laws. Before we look at the ideal gas. Pressure Equation Chem.
From www.zarmtrit.co
pressure force equation force pressure area Sydneycrst Pressure Equation Chem Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Identify the mathematical relationships between the various properties of. Learn and apply boyle’s law. The si unit for. Pressure Equation Chem.
From www.logiota.com
Dalton's Law of Partial Pressure States of Matter Physical Pressure Equation Chem The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Learn what is meant by the term gas laws. Here, p is pressure, k b is boltzmann’s constant, ρ is. Pressure Equation Chem.
From www.youtube.com
CHEMISTRY 101 Calculating pressure volume work YouTube Pressure Equation Chem Pressure is force per unit area of surface; Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Identify the mathematical relationships. Pressure Equation Chem.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Pressure Equation Chem Learn what is meant by the term gas laws. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. The value of the ideal gas constant, r, depends on the. Pressure Equation Chem.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Pressure Equation Chem Learn what is meant by the term gas laws. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. These mean exactly the same thing. By the end of this section, you. Pressure Equation Chem.
From www.youtube.com
Osmotic Pressure Derivation YouTube Pressure Equation Chem The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is. Pressure Equation Chem.
From www.youtube.com
Dalton's Law of Partial Pressure Problems, Mole Fraction, Chemistry Gas Pressure Equation Chem Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). The si value of. Pressure Equation Chem.
From ar.inspiredpencil.com
Pressure Formula Chemistry Pressure Equation Chem Learn what is meant by the term gas laws. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Ideal gas law, relation between the pressure p, volume v, and. Pressure Equation Chem.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Pressure Equation Chem These mean exactly the same thing. The value of the ideal gas constant, r, depends on the other units chosen for the formula. By the end of this section, you will be able to: Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such. Pressure Equation Chem.
From www.youtube.com
Henry's Law Relationship Between Pressure and Solubility of a Gas Pressure Equation Chem The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Identify the mathematical relationships between the various properties of. The. Pressure Equation Chem.
From spmphysics.onlinetuition.com.my
Pressure in Liquid SPM Physics Form 4/Form 5 Revision Notes Pressure Equation Chem Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Learn what is meant by the term gas laws. The value of the ideal gas constant, r, depends on the other units chosen for the formula. Learn and apply boyle’s. Pressure Equation Chem.
From www.youtube.com
Raoults Law and Vapor Pressure Chemistry Tutorial YouTube Pressure Equation Chem The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Identify the mathematical relationships between the various properties of. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better. Pressure Equation Chem.
From gunnargokeodom.blogspot.com
Dalton's Law of Partial Pressure Pressure Equation Chem The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). By the end of this section, you will be able to: Learn what is meant by the term gas laws. These mean exactly the same thing. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Identify the mathematical relationships. Pressure Equation Chem.
From thirdspacelearning.com
Pressure Formula GCSE Maths Steps, Examples & Worksheet Pressure Equation Chem By the end of this section, you will be able to: The value of the ideal gas constant, r, depends on the other units chosen for the formula. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Learn and apply boyle’s law. Identify the mathematical relationships between. Pressure Equation Chem.
From www.expii.com
Charles's Law — Overview & Formula Expii Pressure Equation Chem Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Identify the mathematical relationships between the various properties of. Learn what is meant by the term gas laws. Before we look at the ideal gas equation, let us state the. Pressure Equation Chem.
From socratic.org
If 8 L of a gas at room temperature exerts a pressure of 45 kPa on its Pressure Equation Chem Learn what is meant by the term gas laws. Learn and apply boyle’s law. By the end of this section, you will be able to: The value of the ideal gas constant, r, depends on the other units chosen for the formula. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is. Pressure Equation Chem.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Pressure Equation Chem Pressure is force per unit area of surface; Learn what is meant by the term gas laws. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. These. Pressure Equation Chem.
From sharedocnow.blogspot.com
Pressure Equilibrium Constant Expression sharedoc Pressure Equation Chem The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Before we look at the ideal gas equation, let us. Pressure Equation Chem.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Pressure Equation Chem The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Pressure is force per unit area of surface; Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Here, p is pressure, k b is boltzmann’s constant, ρ is density,. Pressure Equation Chem.
From kayliefernandez.blogspot.com
PreAP Chemistry Units of Pressure/Boyle's Law Pressure Equation Chem Identify the mathematical relationships between the various properties of. Learn what is meant by the term gas laws. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of. Pressure Equation Chem.
From www.youtube.com
CHEM 201 Calculating Vapor Pressure of a NonElectrolyte Solution Pressure Equation Chem By the end of this section, you will be able to: These mean exactly the same thing. Identify the mathematical relationships between the various properties of. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Before we look at. Pressure Equation Chem.
From www.sliderbase.com
Vapor Pressure of Solutions Presentation Chemistry Pressure Equation Chem Identify the mathematical relationships between the various properties of. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Here, p is pressure, k b is boltzmann’s constant, ρ is density, t. Pressure Equation Chem.
From www.youtube.com
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume Pressure Equation Chem The value of the ideal gas constant, r, depends on the other units chosen for the formula. The si value of r is exactly 8.31446261815324 j⋅k −1 ⋅mol −1. Pressure is force per unit area of surface; The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Before we look at the. Pressure Equation Chem.
From spmphysics.onlinetuition.com.my
Pressure in Liquid SPM Physics Form 4/Form 5 Revision Notes Pressure Equation Chem Learn what is meant by the term gas laws. The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Pressure is force per unit area of surface; Learn and apply boyle’s law. Identify the mathematical relationships between the various properties of. By the end of this section, you will be able to:. Pressure Equation Chem.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Pressure Equation Chem Here, p is pressure, k b is boltzmann’s constant, ρ is density, t is absolute temperature, μ is the average particle mass, and m u is the atomic mass constant. Identify the mathematical relationships between the various properties of. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low. Pressure Equation Chem.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Pressure Equation Chem The value of the ideal gas constant, r, depends on the other units chosen for the formula. By the end of this section, you will be able to: The si unit for pressure is the pascal (pa), defined as 1 newton per square meter (n/m 2). Learn what is meant by the term gas laws. Identify the mathematical relationships between. Pressure Equation Chem.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps Pressure Equation Chem Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Before we look at the ideal gas equation, let us state the four gas variables and one constant for a better understanding.. Pressure Equation Chem.