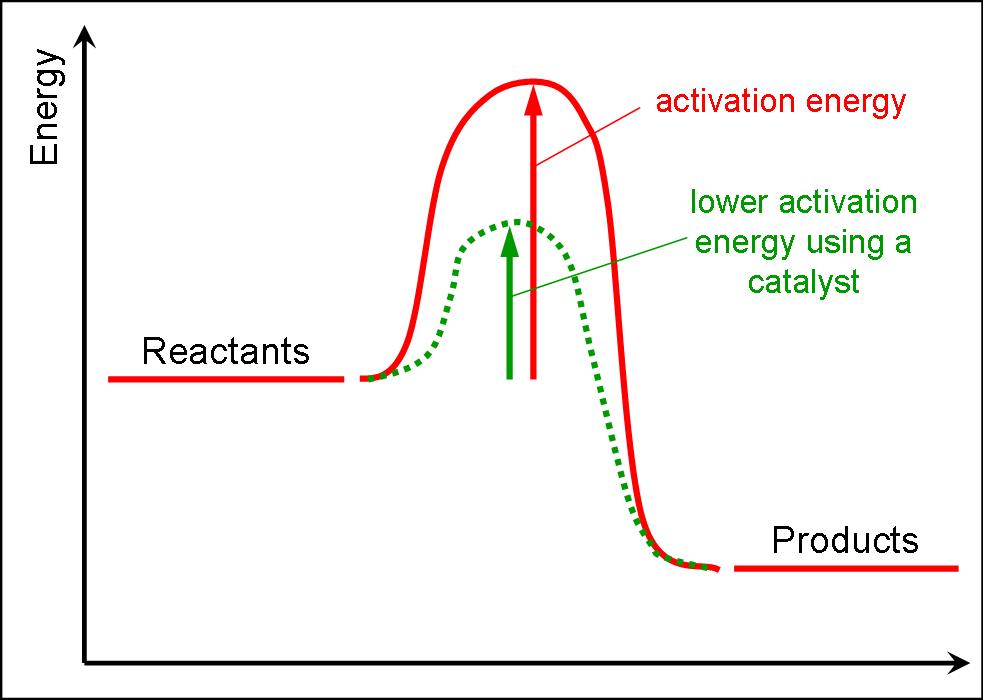
True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation . a catalyst lowers the activation energy of a reaction. A) a catalyst accelerates a reaction by. An enzyme increases the activation energy. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. The concentration of a catalyst may appear in the overall rate equation. Catalysts change the reaction mechanism for a. A catalyst is a substance which increases the rate of a reaction by taking the. Few molecules have enough energy to activate a chemical reaction on their own. Which of the following statements about a catalyst is true? B) a catalyst is chemically unaltered during the chemical reaction and. chemistry questions and answers. Which of the following statements about a catalyst is true? a) a catalyst lowers the activation energy for a reaction. A catalyst accelerates e reaction by changing the amount of.
from btccasting.weebly.com
catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. An enzyme increases the activation energy. Which of the following statements about a catalyst is true? Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. a) a catalyst lowers the activation energy for a reaction. a catalyst lowers the activation energy of a reaction. A catalyst accelerates e reaction by changing the amount of. B) a catalyst is chemically unaltered during the chemical reaction and. chemistry questions and answers.
Enzymes Lower The Activation Energy Of A Reaction btccasting
True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A catalyst accelerates e reaction by changing the amount of. The concentration of a catalyst may appear in the overall rate equation. Few molecules have enough energy to activate a chemical reaction on their own. Catalysts change the reaction mechanism for a. A catalyst accelerates e reaction by changing the amount of. chemistry questions and answers. Which of the following statements about a catalyst is true? Which of the following statements about a catalyst is true? An enzyme increases the activation energy. B) a catalyst is chemically unaltered during the chemical reaction and. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. a) a catalyst lowers the activation energy for a reaction. a catalyst lowers the activation energy of a reaction. A catalyst is a substance which increases the rate of a reaction by taking the. A) a catalyst accelerates a reaction by.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Catalysts change the reaction mechanism for a. The concentration of a catalyst may appear in the overall rate equation. A catalyst is a substance which increases the rate of a reaction by taking the. An enzyme increases the activation energy. A) a catalyst accelerates a reaction by. B) a catalyst is chemically unaltered during the chemical reaction and. a). True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation chemistry questions and answers. a) a catalyst lowers the activation energy for a reaction. A catalyst accelerates e reaction by changing the amount of. Which of the following statements about a catalyst is true? A) a catalyst accelerates a reaction by. a catalyst lowers the activation energy of a reaction. A catalyst is a substance which increases. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. An enzyme increases the activation energy. a) a catalyst lowers the activation energy for a reaction. B) a catalyst is chemically unaltered during the chemical reaction and. chemistry questions and answers. A) a catalyst accelerates a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From philschatz.com
Chemical Reactions · Anatomy and Physiology True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation B) a catalyst is chemically unaltered during the chemical reaction and. A) a catalyst accelerates a reaction by. Catalysts change the reaction mechanism for a. The concentration of a catalyst may appear in the overall rate equation. A catalyst accelerates e reaction by changing the amount of. An enzyme increases the activation energy. Few molecules have enough energy to activate. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From chem.libretexts.org
11.5 Catalytic Hydrogenation Chemistry LibreTexts True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. a catalyst lowers the activation energy of a reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Which of the following statements about a catalyst is. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation An enzyme increases the activation energy. B) a catalyst is chemically unaltered during the chemical reaction and. Which of the following statements about a catalyst is true? A) a catalyst accelerates a reaction by. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. The concentration of a catalyst may appear. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation B) a catalyst is chemically unaltered during the chemical reaction and. Few molecules have enough energy to activate a chemical reaction on their own. Which of the following statements about a catalyst is true? Catalysts change the reaction mechanism for a. a) a catalyst lowers the activation energy for a reaction. An enzyme increases the activation energy. a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From exohcytva.blob.core.windows.net
How Do Enzymes Lower Activation Energy Quizlet at Su Happel blog True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation An enzyme increases the activation energy. Catalysts change the reaction mechanism for a. a) a catalyst lowers the activation energy for a reaction. A) a catalyst accelerates a reaction by. Few molecules have enough energy to activate a chemical reaction on their own. catalysts allow a reaction to proceed via a pathway that has a lower activation energy. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? A catalyst accelerates e reaction by changing the amount of. chemistry questions and answers. a catalyst lowers the activation energy of a reaction. Few molecules have enough energy to activate a chemical reaction on their own. An enzyme increases the activation energy. Which of the following statements about. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From courses.lumenlearning.com
Catalysis Chemistry for Majors True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation B) a catalyst is chemically unaltered during the chemical reaction and. A catalyst accelerates e reaction by changing the amount of. An enzyme increases the activation energy. Few molecules have enough energy to activate a chemical reaction on their own. a) a catalyst lowers the activation energy for a reaction. catalysts allow a reaction to proceed via a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A catalyst is a substance which increases the rate of a reaction by taking the. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. A) a catalyst accelerates a reaction by. chemistry questions and answers. A catalyst accelerates e reaction by changing the amount of. a catalyst lowers. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From dxopopljn.blob.core.windows.net
Enzyme Catalyzed Reactions at Richard Fountain blog True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. A catalyst is a substance which increases the rate of a reaction by taking the. A) a catalyst accelerates a reaction by. Which of the following statements about a catalyst is true? Which of the following statements about a catalyst is. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From schematicrasariopm.z4.web.core.windows.net
Energy Diagram For A Reaction True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? A catalyst is a substance which increases the rate of a reaction by taking the. A catalyst accelerates e reaction by changing the amount of. Few molecules have enough energy to activate a chemical reaction on their own. An enzyme increases the activation energy. A) a catalyst accelerates a reaction. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From 2ly1udq7schematic.z4.web.core.windows.net
Exothermic Potential Energy Diagram Labeled True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. chemistry questions and answers. a catalyst lowers the activation energy of a reaction. An enzyme increases the. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From 2012books.lardbucket.org
Catalysis True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? A catalyst accelerates e reaction by changing the amount of. B) a catalyst is chemically unaltered during the chemical reaction and. Which of the following statements about a catalyst is true? Catalysts change the reaction mechanism for a. Few molecules have enough energy to activate a chemical reaction on their. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Catalysts change the reaction mechanism for a. A) a catalyst accelerates a reaction by. a catalyst lowers the activation energy of a reaction. Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. A catalyst is a substance which increases the rate of a reaction by taking. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation B) a catalyst is chemically unaltered during the chemical reaction and. The concentration of a catalyst may appear in the overall rate equation. An enzyme increases the activation energy. Catalysts change the reaction mechanism for a. chemistry questions and answers. A) a catalyst accelerates a reaction by. A catalyst accelerates e reaction by changing the amount of. a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From studylib.net
Enzymes Lower Activation Energy True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation a catalyst lowers the activation energy of a reaction. Which of the following statements about a catalyst is true? The concentration of a catalyst may appear in the overall rate equation. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. A catalyst accelerates e reaction by changing the amount. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From socratic.org
What are activation energies? Socratic True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Which of the following statements about a catalyst is true? Few molecules have enough energy to activate a chemical reaction on their own. A) a catalyst accelerates a reaction by. a catalyst lowers the activation energy of a reaction. a) a catalyst lowers the activation energy for a reaction. chemistry questions and answers. A catalyst is a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Catalysts change the reaction mechanism for a. Few molecules have enough energy to activate a chemical reaction on their own. The concentration of a catalyst may appear in the overall rate equation. A catalyst accelerates e reaction by changing the amount of. a) a catalyst lowers the activation energy for a reaction. a catalyst lowers the activation energy. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.chim.lu
Activation energy and catalysis True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation a catalyst lowers the activation energy of a reaction. a) a catalyst lowers the activation energy for a reaction. Which of the following statements about a catalyst is true? Catalysts change the reaction mechanism for a. A catalyst is a substance which increases the rate of a reaction by taking the. Few molecules have enough energy to activate. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Few molecules have enough energy to activate a chemical reaction on their own. A catalyst accelerates e reaction by changing the amount of. A catalyst is a substance which increases the rate of a reaction by taking the. chemistry questions and answers. An enzyme increases the activation energy. B) a catalyst is chemically unaltered during the chemical reaction and.. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A catalyst accelerates e reaction by changing the amount of. Which of the following statements about a catalyst is true? A) a catalyst accelerates a reaction by. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. An enzyme increases the activation energy. chemistry questions and answers. B) a catalyst. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Catalysts change the reaction mechanism for a. Which of the following statements about a catalyst is true? a catalyst lowers the activation energy of a reaction. A) a catalyst accelerates a reaction by. The concentration of a catalyst may appear in the overall rate equation. Which of the following statements about a catalyst is true? chemistry questions and. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From brainly.com
Classify each statement about catalysts as true or false. Catalyst True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation An enzyme increases the activation energy. a) a catalyst lowers the activation energy for a reaction. A catalyst is a substance which increases the rate of a reaction by taking the. a catalyst lowers the activation energy of a reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation chemistry questions and answers. B) a catalyst is chemically unaltered during the chemical reaction and. a catalyst lowers the activation energy of a reaction. A) a catalyst accelerates a reaction by. a) a catalyst lowers the activation energy for a reaction. The concentration of a catalyst may appear in the overall rate equation. Catalysts change the reaction. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A catalyst is a substance which increases the rate of a reaction by taking the. A catalyst accelerates e reaction by changing the amount of. Catalysts change the reaction mechanism for a. a) a catalyst lowers the activation energy for a reaction. Few molecules have enough energy to activate a chemical reaction on their own. catalysts allow a. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From morioh.com
Collision Theory Arrhenius Equation & Activation Energy Chemical True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation Few molecules have enough energy to activate a chemical reaction on their own. a catalyst lowers the activation energy of a reaction. Catalysts change the reaction mechanism for a. A catalyst accelerates e reaction by changing the amount of. A) a catalyst accelerates a reaction by. Which of the following statements about a catalyst is true? catalysts allow. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation a catalyst lowers the activation energy of a reaction. B) a catalyst is chemically unaltered during the chemical reaction and. Which of the following statements about a catalyst is true? Few molecules have enough energy to activate a chemical reaction on their own. An enzyme increases the activation energy. A catalyst accelerates e reaction by changing the amount of.. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation a catalyst lowers the activation energy of a reaction. A catalyst is a substance which increases the rate of a reaction by taking the. A) a catalyst accelerates a reaction by. Which of the following statements about a catalyst is true? Which of the following statements about a catalyst is true? B) a catalyst is chemically unaltered during the. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From present5.com
Enzyme Structure classification and mechanism of action True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation chemistry questions and answers. a) a catalyst lowers the activation energy for a reaction. A catalyst accelerates e reaction by changing the amount of. The concentration of a catalyst may appear in the overall rate equation. Catalysts change the reaction mechanism for a. catalysts allow a reaction to proceed via a pathway that has a lower activation. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation An enzyme increases the activation energy. Catalysts change the reaction mechanism for a. Which of the following statements about a catalyst is true? B) a catalyst is chemically unaltered during the chemical reaction and. A catalyst accelerates e reaction by changing the amount of. a) a catalyst lowers the activation energy for a reaction. catalysts allow a reaction. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A) a catalyst accelerates a reaction by. The concentration of a catalyst may appear in the overall rate equation. Which of the following statements about a catalyst is true? chemistry questions and answers. a) a catalyst lowers the activation energy for a reaction. Few molecules have enough energy to activate a chemical reaction on their own. A catalyst. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A catalyst accelerates e reaction by changing the amount of. chemistry questions and answers. a) a catalyst lowers the activation energy for a reaction. Few molecules have enough energy to activate a chemical reaction on their own. a catalyst lowers the activation energy of a reaction. catalysts allow a reaction to proceed via a pathway that. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.
From dbcarnahanoutmantles.z21.web.core.windows.net
Two Step Exothermic Reaction Diagram True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation A) a catalyst accelerates a reaction by. a) a catalyst lowers the activation energy for a reaction. The concentration of a catalyst may appear in the overall rate equation. A catalyst is a substance which increases the rate of a reaction by taking the. B) a catalyst is chemically unaltered during the chemical reaction and. Which of the following. True Or False A Catalyst Accelerates A Reaction By Lowering The Energy Of Activation.