Is Acetone Non Polar . There are different degrees of polarity, and acetone is. as both water and methanol are liquids, the word miscible can be used in place of soluble. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Sodium sulfate is an ionic. is acetone polar or nonpolar? Because of the carbonyl group, acetone is a somewhat polar molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not.
from hxevakmnb.blob.core.windows.net
Sodium sulfate is an ionic. There are different degrees of polarity, and acetone is. Polarity is the phenomenon where charges are separated to form an electric dipole moment. as both water and methanol are liquids, the word miscible can be used in place of soluble. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. Because of the carbonyl group, acetone is a somewhat polar molecule. is acetone polar or nonpolar? 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms.
Is Hexane Acetone Polar Or Nonpolar at Lisa Boyd blog
Is Acetone Non Polar is acetone polar or nonpolar? acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. as both water and methanol are liquids, the word miscible can be used in place of soluble. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Sodium sulfate is an ionic. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is. is acetone polar or nonpolar?
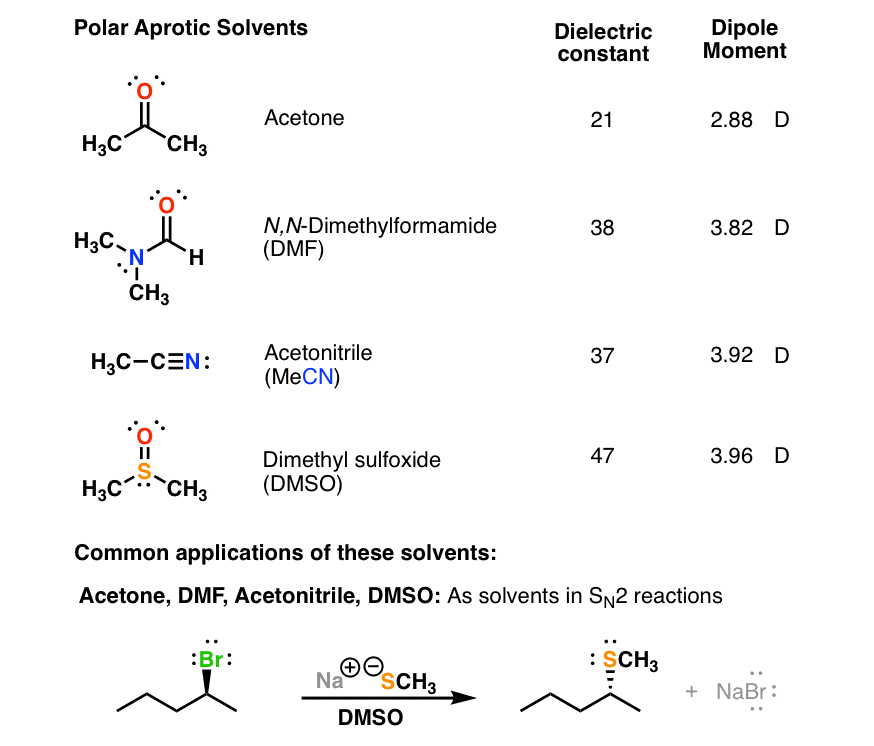
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Non Polar acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is. as both water and methanol. Is Acetone Non Polar.
From www.numerade.com
SOLVED List the following from most polar to least polar Acetone Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. is acetone polar or nonpolar? Sodium sulfate is an ionic. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Polarity is the phenomenon where charges are separated to form an. Is Acetone Non Polar.
From www.youtube.com
Is acetone polar or nonpolar? Will NaCl dissolve in it? YouTube Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. Because of the carbonyl group, acetone is a somewhat polar molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Learn how to draw the lewis structure and check. Is Acetone Non Polar.
From www.youtube.com
Acetone Lewis Structure How to Draw the Lewis Structure for Acetone Is Acetone Non Polar acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. There are different degrees of polarity, and acetone is. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Polarity is the phenomenon where charges are separated to. Is Acetone Non Polar.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents Is Acetone Non Polar Polarity is the phenomenon where charges are separated to form an electric dipole moment. as both water and methanol are liquids, the word miscible can be used in place of soluble. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. 23 rows however, acetone is still considered. Is Acetone Non Polar.
From www.numerade.com
Solvent Solvent Polarity Index, P Hexane 0.1 Carbon tetrachloride Is Acetone Non Polar acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Sodium sulfate is an ionic. There are different degrees of polarity, and acetone is. as both water and methanol are liquids, the word miscible can. Is Acetone Non Polar.
From studiousguy.com
Acetone Uses in Daily Life StudiousGuy Is Acetone Non Polar Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. Sodium sulfate is an ionic. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. acetone (c3h6o) is a polar molecule because the oxygen atom is more. Is Acetone Non Polar.
From www.youtube.com
Is C3H6O (Acetone) Polar or NonPolar? YouTube Is Acetone Non Polar 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. is acetone polar or nonpolar? as both water and methanol are liquids, the word miscible can be used in place of soluble. Polarity is the phenomenon where charges are separated to form an electric dipole moment.. Is Acetone Non Polar.
From julianajoysgraves.blogspot.com
Acetone Polar or Nonpolar Solvent JulianajoysGraves Is Acetone Non Polar 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Because of the carbonyl group, acetone is a somewhat polar molecule. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Polarity is the phenomenon where charges are. Is Acetone Non Polar.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. Polarity is the phenomenon where charges are separated to form an electric dipole moment. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Because of the carbonyl group, acetone. Is Acetone Non Polar.
From www.chegg.com
Solved Based on the structures provide for ethanol, water, Is Acetone Non Polar Polarity is the phenomenon where charges are separated to form an electric dipole moment. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. as both water and methanol are liquids, the word miscible can be used in place of soluble. is acetone polar or nonpolar? 23. Is Acetone Non Polar.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. is acetone polar or nonpolar? Because of the carbonyl group, acetone is a somewhat polar molecule. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Sodium sulfate is an ionic. There are different degrees of polarity,. Is Acetone Non Polar.
From www.chegg.com
Solved An electrostatic potential map for acetone (CH3)2CO), Is Acetone Non Polar 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. as both water and methanol are liquids, the word miscible can be used in place of soluble. is acetone polar or nonpolar? Learn how to draw the lewis structure and check the bond polarity and molecular. Is Acetone Non Polar.
From www.techno-science.net
Acétone Définition et Explications Is Acetone Non Polar Sodium sulfate is an ionic. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. There are different degrees of polarity, and acetone is. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Learn how to draw. Is Acetone Non Polar.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Is Acetone Non Polar Sodium sulfate is an ionic. Polarity is the phenomenon where charges are separated to form an electric dipole moment. There are different degrees of polarity, and acetone is. Because of the carbonyl group, acetone is a somewhat polar molecule. is acetone polar or nonpolar? as both water and methanol are liquids, the word miscible can be used in. Is Acetone Non Polar.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Is Acetone Non Polar Polarity is the phenomenon where charges are separated to form an electric dipole moment. is acetone polar or nonpolar? as both water and methanol are liquids, the word miscible can be used in place of soluble. Sodium sulfate is an ionic. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone. Is Acetone Non Polar.
From study.com
Acetone Structure, Properties & Uses Lesson Is Acetone Non Polar is acetone polar or nonpolar? acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. There are different degrees of polarity, and acetone is. Sodium sulfate is an ionic. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Learn how to draw the lewis. Is Acetone Non Polar.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone Non Polar Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. Because of the carbonyl group, acetone is a somewhat polar molecule. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. 23 rows however, acetone is still considered a. Is Acetone Non Polar.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of. Is Acetone Non Polar.
From mungfali.com
Acetone Polar Or Nonpolar Is Acetone Non Polar Sodium sulfate is an ionic. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. Because of the carbonyl group, acetone is a somewhat polar molecule. as both water and methanol are liquids, the word miscible can be used in place of soluble. acetone (c3h6o) is a polar. Is Acetone Non Polar.
From smakbo.github.io
Acetone Physical Properties of Acetone Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. is acetone polar or nonpolar? Sodium sulfate is an ionic. Because of the carbonyl group, acetone is a somewhat. Is Acetone Non Polar.
From www.differencebetween.com
Difference Between Acetone and Isopropyl Alcohol Compare the Is Acetone Non Polar Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. as both water and methanol are liquids, the word miscible can be used in place of soluble. Because of the carbonyl group, acetone is a somewhat polar molecule. 23 rows however, acetone is still considered a polar aprotic. Is Acetone Non Polar.
From assolea.org
Formule de l'acétone Définition, concepts et exemples Association LEA Is Acetone Non Polar is acetone polar or nonpolar? Sodium sulfate is an ionic. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Polarity is the phenomenon where charges are. Is Acetone Non Polar.
From ar.inspiredpencil.com
Acetone Structure Is Acetone Non Polar Polarity is the phenomenon where charges are separated to form an electric dipole moment. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Sodium sulfate is an ionic. as both water and methanol are liquids, the word miscible can be used in place of soluble. . Is Acetone Non Polar.
From it.wikipedia.org
Acetone Wikipedia Is Acetone Non Polar Because of the carbonyl group, acetone is a somewhat polar molecule. Sodium sulfate is an ionic. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Learn how to draw the lewis structure and check the bond polarity and molecular geometry of acetone and other molecules. There are different degrees of polarity, and acetone is. . Is Acetone Non Polar.
From www.youtube.com
a mixture of ethanol and acetone shows positive deviation YouTube Is Acetone Non Polar Sodium sulfate is an ionic. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Polarity is the phenomenon where charges are separated to form an electric dipole. Is Acetone Non Polar.
From www.youtube.com
Why does acetone dissolve both polar and nonpolar? YouTube Is Acetone Non Polar There are different degrees of polarity, and acetone is. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. acetone (c3h6o) is. Is Acetone Non Polar.
From www.youtube.com
Is C3H6O Polar or NonPolar? (Acetone) YouTube Is Acetone Non Polar There are different degrees of polarity, and acetone is. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. is acetone polar or nonpolar? as both water and methanol are liquids, the word miscible can be used in place of soluble. 23 rows however, acetone is still. Is Acetone Non Polar.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Is Acetone Non Polar as both water and methanol are liquids, the word miscible can be used in place of soluble. Because of the carbonyl group, acetone is a somewhat polar molecule. Polarity is the phenomenon where charges are separated to form an electric dipole moment. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon. Is Acetone Non Polar.
From hxevakmnb.blob.core.windows.net
Is Hexane Acetone Polar Or Nonpolar at Lisa Boyd blog Is Acetone Non Polar There are different degrees of polarity, and acetone is. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. Polarity is the phenomenon where charges are separated to. Is Acetone Non Polar.
From www.coursehero.com
[Solved] Name 2. a. Draw the Lewis structure of acetone, (CH3)2CO (C in Is Acetone Non Polar is acetone polar or nonpolar? acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than the carbon and hydrogen atoms. There are different degrees of polarity, and acetone is. as both water and methanol are liquids, the word miscible can be used in place of soluble. 23 rows however, acetone is still. Is Acetone Non Polar.
From www.youtube.com
Intermolecular Forces for (CH3)2CO Acetone YouTube Is Acetone Non Polar Sodium sulfate is an ionic. There are different degrees of polarity, and acetone is. Polarity is the phenomenon where charges are separated to form an electric dipole moment. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Learn how to draw the lewis structure and check the. Is Acetone Non Polar.
From www.youtube.com
Is acetone a polar or non polar solvent? YouTube Is Acetone Non Polar 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Because of the carbonyl group, acetone is a somewhat polar molecule. is acetone polar or nonpolar? as both water and methanol are liquids, the word miscible can be used in place of soluble. Learn how to. Is Acetone Non Polar.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Is Acetone Non Polar Because of the carbonyl group, acetone is a somewhat polar molecule. is acetone polar or nonpolar? There are different degrees of polarity, and acetone is. Sodium sulfate is an ionic. Polarity is the phenomenon where charges are separated to form an electric dipole moment. acetone (c3h6o) is a polar molecule because the oxygen atom is more electronegative than. Is Acetone Non Polar.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone Non Polar There are different degrees of polarity, and acetone is. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Sodium sulfate is an ionic. as both water and methanol are liquids, the word miscible can be used in place of soluble. is acetone polar or nonpolar? Because of the carbonyl group, acetone is a. Is Acetone Non Polar.