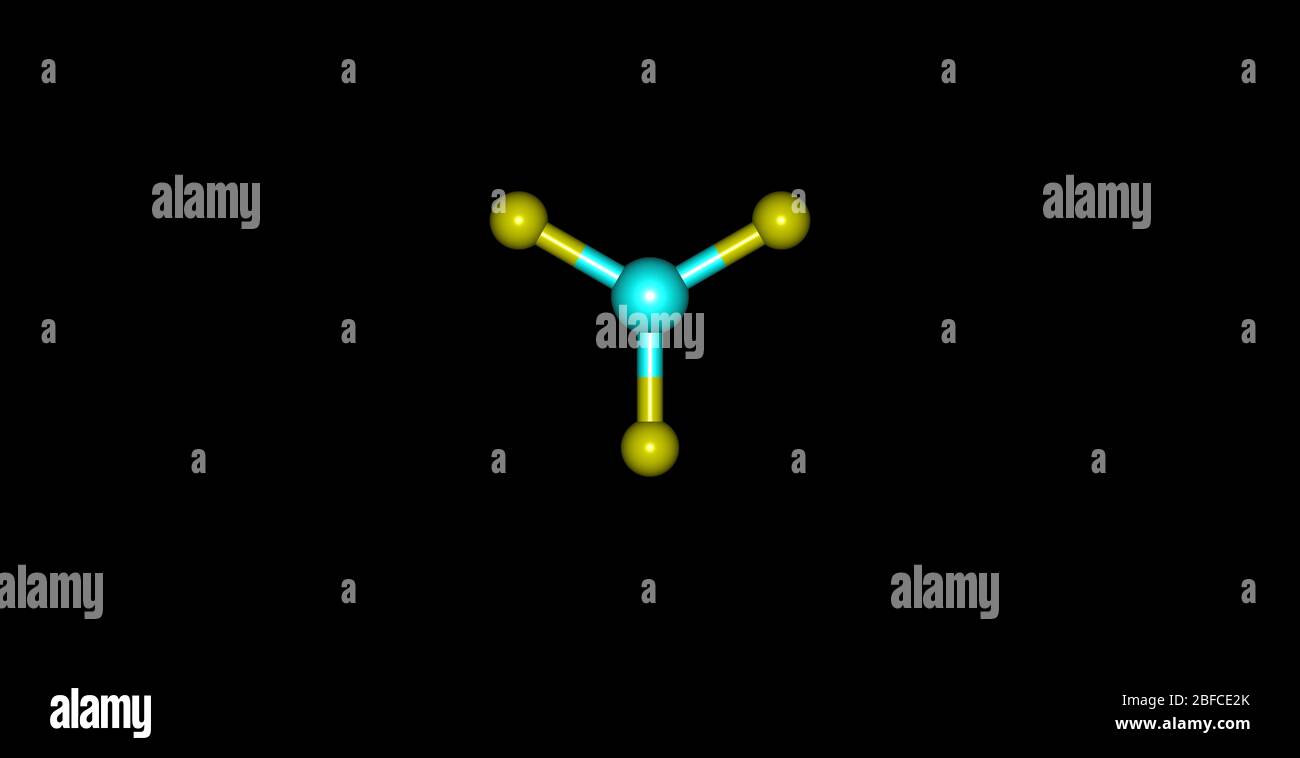
Chlorine Trifluoride Molecule Structure . It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. Chlorine trifluoride has been used in a variety of applications since it was first discovered. It is an interhalogen compound. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. It reacts with water to form chlorine and hydrofluoric acid with release of. The chemical formula clf3 represents chlorine trifluoride. Advanced this is the new chemspider site, some advanced search features are still to be released. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. The properties of chlorine trifluoride. The compound is highly reactive, poisonous, and corrosive.
from www.alamy.com
The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The properties of chlorine trifluoride. It reacts with water to form chlorine and hydrofluoric acid with release of. The chemical formula clf3 represents chlorine trifluoride. The compound is highly reactive, poisonous, and corrosive. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature.
Chlorine trifluoride is an interhalogen compound with the formula ClF3
Chlorine Trifluoride Molecule Structure It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. It reacts with water to form chlorine and hydrofluoric acid with release of. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. The chemical formula clf3 represents chlorine trifluoride. Advanced this is the new chemspider site, some advanced search features are still to be released. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. It is an interhalogen compound. The properties of chlorine trifluoride. Chlorine trifluoride has been used in a variety of applications since it was first discovered. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. The compound is highly reactive, poisonous, and corrosive. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Molecule Structure Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. The properties of chlorine trifluoride. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. The compound is highly reactive, poisonous, and corrosive. It reacts with water to form chlorine and hydrofluoric. Chlorine Trifluoride Molecule Structure.
From imgbin.com
Nitrogen Trifluoride Molecule Ballandstick Model Trigonal Pyramidal Chlorine Trifluoride Molecule Structure The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. It is an interhalogen compound. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Chlorine trifluoride has been used in a variety of applications since it was first discovered. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen.. Chlorine Trifluoride Molecule Structure.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Molecule Structure Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. It is an interhalogen compound. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Advanced this is the new chemspider site, some advanced search features are still to be released. It reacts with water to form chlorine. Chlorine Trifluoride Molecule Structure.
From www.istockphoto.com
Chlorine Trifluoride Molecular Structure Isolated On White Stock Photo Chlorine Trifluoride Molecule Structure It is an interhalogen compound. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. The compound is highly reactive, poisonous, and corrosive. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,.. Chlorine Trifluoride Molecule Structure.
From www.youtube.com
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube Chlorine Trifluoride Molecule Structure Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. It is an interhalogen compound. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical. Chlorine Trifluoride Molecule Structure.
From joipbrcxt.blob.core.windows.net
Chlorine Trifluoride Is Polar Molecule at Sue Hinds blog Chlorine Trifluoride Molecule Structure The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. The chemical formula clf3 represents chlorine trifluoride. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. It is an interhalogen compound. Advanced this. Chlorine Trifluoride Molecule Structure.
From www.chemistry-reference.com
CHLORINE TRIFLUORIDE (7790912) Physical Properties • Chemical Chlorine Trifluoride Molecule Structure It is an interhalogen compound. The chemical formula clf3 represents chlorine trifluoride. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. It reacts with water to form chlorine and hydrofluoric acid with release of. The compound is highly reactive, poisonous, and corrosive. The chlorine. Chlorine Trifluoride Molecule Structure.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Molecule Structure The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Advanced this is the new. Chlorine Trifluoride Molecule Structure.
From www.istockphoto.com
Chlorine Trifluoride Molecular Structure Isolated On Grey Stock Photo Chlorine Trifluoride Molecule Structure It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. It reacts with water to form chlorine and hydrofluoric acid with release of. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. The chemical formula clf3. Chlorine Trifluoride Molecule Structure.
From www.kindpng.com
Chlorine Trifluoride 3d Balls Chlorine Trifluoride 3d Structure, HD Chlorine Trifluoride Molecule Structure It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. The properties of chlorine trifluoride. Advanced this is the new chemspider site, some advanced search features are still to be released. The chemical formula clf3 represents chlorine. Chlorine Trifluoride Molecule Structure.
From www.22measured.com
SOLVED The reaction of hydrazine (N2H4) and chlorine trifluoride (ClF3 Chlorine Trifluoride Molecule Structure It is an interhalogen compound. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. The compound is highly reactive, poisonous, and corrosive. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. It reacts with water to form chlorine and hydrofluoric acid with release of. 3 lone pairs are present. Chlorine Trifluoride Molecule Structure.
From alchetron.com
Chlorine trifluoride Alchetron, The Free Social Encyclopedia Chlorine Trifluoride Molecule Structure The chemical formula clf3 represents chlorine trifluoride. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. Chlorine trifluoride has been used in a variety of applications since it was first discovered. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. Chlorine trifluoride is a chemical. Chlorine Trifluoride Molecule Structure.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Molecule Structure It reacts with water to form chlorine and hydrofluoric acid with release of. Advanced this is the new chemspider site, some advanced search features are still to be released. Chlorine trifluoride has been used in a variety of applications since it was first discovered. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique. Chlorine Trifluoride Molecule Structure.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Molecule Structure The properties of chlorine trifluoride. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride has been used in a variety of applications since it was first discovered. It is an interhalogen compound. The chemical formula clf3 represents chlorine trifluoride. Chlorine trifluoride appears as a colorless gas or green. Chlorine Trifluoride Molecule Structure.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Molecule Structure It is an interhalogen compound. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Chlorine trifluoride has been used in a variety of applications since it was. Chlorine Trifluoride Molecule Structure.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Molecule Structure The chemical formula clf3 represents chlorine trifluoride. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride is a chemical compound. Chlorine Trifluoride Molecule Structure.
From joivbtcfv.blob.core.windows.net
Chemical Formula Of Chlorine Trifluoride at Tom Coble blog Chlorine Trifluoride Molecule Structure Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. The compound is highly reactive, poisonous, and corrosive. It reacts with water to form chlorine and hydrofluoric acid with release of. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The chlorine trifluoride (clf 3) molecule has a. Chlorine Trifluoride Molecule Structure.
From www.dreamstime.com
Chlorine molecule stock illustration. Illustration of biotech 3900921 Chlorine Trifluoride Molecule Structure 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. It reacts. Chlorine Trifluoride Molecule Structure.
From www.dreamstime.com
Chlorine Trifluoride Gas Molecule Icon Stock Illustration Chlorine Trifluoride Molecule Structure The compound is highly reactive, poisonous, and corrosive. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. It reacts with water to form chlorine and hydrofluoric acid with release of. Advanced this is the new chemspider. Chlorine Trifluoride Molecule Structure.
From www.youtube.com
Molecular Structure of Chlorine Trifluoride Using VSEPR Theory Nature Chlorine Trifluoride Molecule Structure The compound is highly reactive, poisonous, and corrosive. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. It is an interhalogen compound. Advanced this is the new chemspider site, some advanced search features are still to be released. The chlorine trifluoride (clf 3) molecule has a total. Chlorine Trifluoride Molecule Structure.
From joipbrcxt.blob.core.windows.net
Chlorine Trifluoride Is Polar Molecule at Sue Hinds blog Chlorine Trifluoride Molecule Structure Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. It reacts with water to form chlorine and hydrofluoric acid with release of. It is an interhalogen compound. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several. Chlorine Trifluoride Molecule Structure.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Trifluoride Molecule Structure Advanced this is the new chemspider site, some advanced search features are still to be released. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. The properties of chlorine trifluoride. It reacts with water to form chlorine and hydrofluoric acid with release of. It dissolves in water forms hydrogen fluoride, hydrogen chloride and. Chlorine Trifluoride Molecule Structure.
From www.youtube.com
ClF3 Molecular Geometry, Bond Angles & Electron Geometry (Chlorine Chlorine Trifluoride Molecule Structure Chlorine trifluoride has been used in a variety of applications since it was first discovered. The compound is highly reactive, poisonous, and corrosive. The chemical formula clf3 represents chlorine trifluoride. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. It reacts with water to form chlorine and hydrofluoric acid with release of. It is an interhalogen. Chlorine Trifluoride Molecule Structure.
From byjus.com
Hybridization of ClF3 Hybridization of Cl in Chlorine Trifluoride Chlorine Trifluoride Molecule Structure Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. The properties of chlorine trifluoride. It reacts with water to form chlorine and hydrofluoric acid with release of. Chlorine trifluoride appears as. Chlorine Trifluoride Molecule Structure.
From chemicalportal.ru
Фторид хлора (III) — свойства, получение и применение Chlorine Trifluoride Molecule Structure The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Chlorine trifluoride has been used in a variety of applications since it was first discovered. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. It reacts with water to. Chlorine Trifluoride Molecule Structure.
From slideplayer.com
Molecular Geometry. ppt download Chlorine Trifluoride Molecule Structure Chlorine trifluoride has been used in a variety of applications since it was first discovered. It is an interhalogen compound. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. The chemical formula clf3 represents chlorine trifluoride. The compound is highly reactive, poisonous, and corrosive. Chlorine trifluoride or clf3 is an extremely reactive chemical. Chlorine Trifluoride Molecule Structure.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Trifluoride Molecule Structure It is an interhalogen compound. Chlorine trifluoride has been used in a variety of applications since it was first discovered. Advanced this is the new chemspider site, some advanced search features are still to be released. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Chlorine trifluoride or clf3 is an extremely reactive. Chlorine Trifluoride Molecule Structure.
From www.pngwing.com
Chlorine pentafluoride Xenon oxytetrafluoride Chlorine trifluoride Chlorine Trifluoride Molecule Structure It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. It is an interhalogen compound. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on the central chlorine (cl) atom,. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. The chlorine trifluoride (clf 3) molecule has a. Chlorine Trifluoride Molecule Structure.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Chlorine Trifluoride Molecule Structure It is an interhalogen compound. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied. Chlorine Trifluoride Molecule Structure.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Trifluoride Molecule Structure Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. Chlorine trifluoride has been used in a variety of applications since it was first discovered. The chemical formula clf3 represents chlorine trifluoride. The properties of chlorine trifluoride. It reacts with. Chlorine Trifluoride Molecule Structure.
From wirelistetiquette.z13.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Trifluoride Molecule Structure The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. The properties of chlorine trifluoride. It is an interhalogen compound. The chemical formula clf3 represents chlorine trifluoride. It reacts with water to form chlorine and hydrofluoric acid with release of. 3 lone pairs are present on each fluorine (f) atom while 2 lone pairs are situated on. Chlorine Trifluoride Molecule Structure.
From www.gauthmath.com
Solved What is the formula of this binary molecular compound? chlorine Chlorine Trifluoride Molecule Structure Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and. The chlorine trifluoride (clf 3) molecule has a total of 11 lone pairs. Advanced this is the new chemspider site, some advanced search features are still to be released. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. 3 lone. Chlorine Trifluoride Molecule Structure.
From www.alamy.com
Chlorine gas white background hires stock photography and images Alamy Chlorine Trifluoride Molecule Structure Chlorine trifluoride has been used in a variety of applications since it was first discovered. The compound is highly reactive, poisonous, and corrosive. The chemical formula clf3 represents chlorine trifluoride. Advanced this is the new chemspider site, some advanced search features are still to be released. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor.. Chlorine Trifluoride Molecule Structure.
From www.istockphoto.com
Chlorine Trifluoride Molecular Structure Isolated On White Stock Photo Chlorine Trifluoride Molecule Structure The compound is highly reactive, poisonous, and corrosive. It reacts with water to form chlorine and hydrofluoric acid with release of. The chemical formula clf3 represents chlorine trifluoride. It is an interhalogen compound. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. 3 lone pairs are present on each fluorine (f) atom while. Chlorine Trifluoride Molecule Structure.
From www.alamy.com
Chlorine trifluoride hires stock photography and images Alamy Chlorine Trifluoride Molecule Structure The properties of chlorine trifluoride. The chemical formula clf3 represents chlorine trifluoride. Chlorine trifluoride has been used in a variety of applications since it was first discovered. Chlorine trifluoride is a chemical compound that is not present as a free compound in nature. It dissolves in water forms hydrogen fluoride, hydrogen chloride and oxygen. Chlorine trifluoride or clf3 is an. Chlorine Trifluoride Molecule Structure.