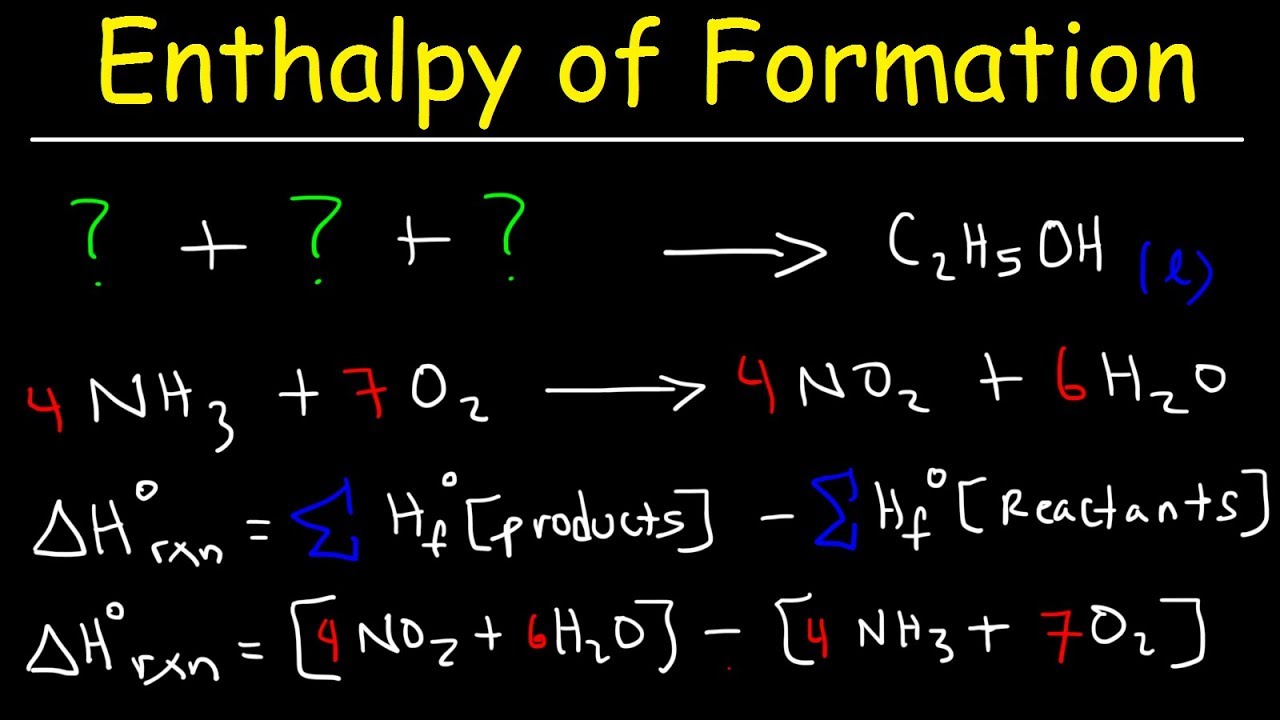
Standard Heat Of Formation Tutorial . Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Start practicing—and saving your progress—now:. Courses on khan academy are always 100% free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that.
from printablevascelomgm.z13.web.core.windows.net
An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. Courses on khan academy are always 100% free. Write the general equation for calculating the standard enthalpy of reaction: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Start practicing—and saving your progress—now:.
How To Determine The Heat Of Formation
Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Start practicing—and saving your progress—now:. Courses on khan academy are always 100% free. Write the general equation for calculating the standard enthalpy of reaction: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law.
From www.chegg.com
Solved The standard heat of formation, ДНі , is defined as Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Courses on khan academy are always 100% free. An application of hess's. Standard Heat Of Formation Tutorial.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Write the general equation for calculating the standard enthalpy of reaction: Start practicing—and saving your progress—now:. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Heat Of Formation Tutorial.
From slideplayer.com
Heats of Formation. ppt download Standard Heat Of Formation Tutorial An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Now that we've looked at various ways to determine enthalpy changes for a. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT CHAPTER 4 PowerPoint Presentation, free download ID3180471 Standard Heat Of Formation Tutorial The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Δ rho = σδ fho (products) − σδ fho (reactants) step 3:. Standard Heat Of Formation Tutorial.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Courses on khan academy are always 100% free. An introduction to using. Standard Heat Of Formation Tutorial.
From kunduz.com
[ANSWERED] Using standard heats of formation, calcu... Standard Heat Of Formation Tutorial Δ rho = σδ fho (products) − σδ fho (reactants) step 3: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Courses on khan academy are always 100% free. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to. Standard Heat Of Formation Tutorial.
From www.youtube.com
Heat of formation Thermodynamics Chemistry Khan Academy YouTube Standard Heat Of Formation Tutorial Courses on khan academy are always 100% free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Write the general. Standard Heat Of Formation Tutorial.
From www.chegg.com
Solved 5. Use standard heats of formation (table on last Standard Heat Of Formation Tutorial Start practicing—and saving your progress—now:. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Write the general equation for calculating. Standard Heat Of Formation Tutorial.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Courses on khan academy are always 100% free. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Start practicing—and saving your progress—now:.. Standard Heat Of Formation Tutorial.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. Δ rho. Standard Heat Of Formation Tutorial.
From www.youtube.com
Standard Heat of Formation & Calculating Standard Heat of Reaction Standard Heat Of Formation Tutorial Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Start practicing—and saving your progress—now:. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction. Standard Heat Of Formation Tutorial.
From slideplayer.com
Heats of Formation. ppt download Standard Heat Of Formation Tutorial An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Start practicing—and saving your progress—now:. Now that we've looked at various ways. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Heat Of Formation Tutorial Δ rho = σδ fho (products) − σδ fho (reactants) step 3: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law.. Standard Heat Of Formation Tutorial.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Courses on khan academy are always 100% free. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: An application of hess's. Standard Heat Of Formation Tutorial.
From www.youtube.com
Standard Heat of Formation YouTube Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Courses on khan academy are always 100% free. The standard enthalpy of formation. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download Standard Heat Of Formation Tutorial An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Heat Of Formation Tutorial.
From storage.googleapis.com
Standard Enthalpy Of Formation Sulfuric Acid Standard Heat Of Formation Tutorial The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Start practicing—and saving your progress—now:. Courses on khan. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Tutorial The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Courses on khan academy are always 100% free. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. An application of. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Now that we've looked at various ways to determine enthalpy changes for a. Standard Heat Of Formation Tutorial.
From mungfali.com
Standard Heat Formation Chart Standard Heat Of Formation Tutorial The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's. Standard Heat Of Formation Tutorial.
From www.coursehero.com
[Solved] Which of the substances have a standard heat of formation Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. An application of hess's law allows us to use standard heats. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation Tutorial Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Write the general equation for calculating the standard enthalpy of reaction: Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. Courses on khan academy are always 100% free. An introduction to using standard heat of formation, continued in lesson 10.7b as a. Standard Heat Of Formation Tutorial.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Heat Of Formation Tutorial Courses on khan academy are always 100% free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. An introduction to using standard heat of formation, continued in lesson 10.7b as a. Standard Heat Of Formation Tutorial.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. Start practicing—and saving your progress—now:. The standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Heat Of Formation Tutorial.
From slideplayer.com
Hermochemistry. ppt download Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Now that we've looked at various ways to determine enthalpy changes for a particular reaction,. Start practicing—and saving your progress—now:. An application of hess's. Standard Heat Of Formation Tutorial.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Tutorial Start practicing—and saving your progress—now:. Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Heat Of Formation Tutorial.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Tutorial The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Δ. Standard Heat Of Formation Tutorial.
From www.chegg.com
Solved Use and interpret standard heats of formation. (a) Standard Heat Of Formation Tutorial An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: Courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. The standard enthalpy of formation, δh of, is the enthalpy change. Standard Heat Of Formation Tutorial.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard Standard Heat Of Formation Tutorial An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. Write the general equation for calculating the standard enthalpy of reaction: Courses on khan academy are always 100% free. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation. Standard Heat Of Formation Tutorial.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Tutorial An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. Write the general equation for calculating the standard enthalpy of reaction: The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. An application. Standard Heat Of Formation Tutorial.
From www.chegg.com
Solved The standard heat of formation for CaCl2 (s) is −796 Standard Heat Of Formation Tutorial Start practicing—and saving your progress—now:. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances. Standard Heat Of Formation Tutorial.
From printablevascelomgm.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Tutorial An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. Courses on khan academy are always 100% free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation. Standard Heat Of Formation Tutorial.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Of Formation Tutorial The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. Courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law.. Standard Heat Of Formation Tutorial.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Tutorial Write the general equation for calculating the standard enthalpy of reaction: An introduction to using standard heat of formation, continued in lesson 10.7b as a method to calculate heat of reaction along with hess's law. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions.. Standard Heat Of Formation Tutorial.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Of Formation Tutorial Courses on khan academy are always 100% free. An application of hess's law allows us to use standard heats of formation to indirectly calculate the heat of reaction for any reaction that. Δ rho = σδ fho (products) − σδ fho (reactants) step 3: The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Heat Of Formation Tutorial.