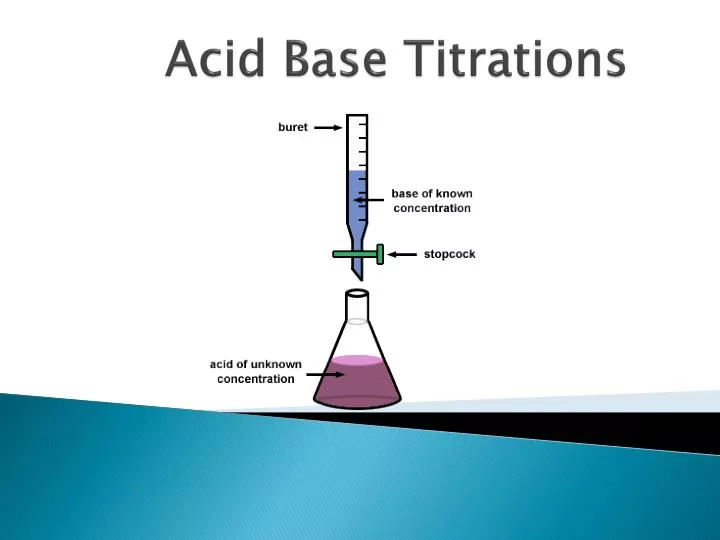
Titration In Pharmaceutical Chemistry . Titration of a base with a standard acid. If you are in the. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. The importance of titrations in pharmaceutical analysis. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of an acid with a standard based. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Understand the difference between manual titration and autotitration for pharmaceutical samples. Sodium hydroxide with hydrochloric acid. Learn how an electrode works and its.
from mungfali.com
Naoh + hcl → nacl+h2o. The importance of titrations in pharmaceutical analysis. Sodium hydroxide with hydrochloric acid. If you are in the. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration of a base with a standard acid. Titration of an acid with a standard based. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in.
Acid Base Titration Experiment
Titration In Pharmaceutical Chemistry Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. The importance of titrations in pharmaceutical analysis. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration of a base with a standard acid. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. If you are in the. Learn how an electrode works and its. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Sodium hydroxide with hydrochloric acid. Titration of an acid with a standard based. Naoh + hcl → nacl+h2o. Understand the difference between manual titration and autotitration for pharmaceutical samples.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. Understand the difference between manual titration and autotitration for pharmaceutical samples. If you are in the. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Titration of a base with a standard acid. Sodium hydroxide with hydrochloric acid. Titration of an acid with a. Titration In Pharmaceutical Chemistry.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration In Pharmaceutical Chemistry Sodium hydroxide with hydrochloric acid. Understand the difference between manual titration and autotitration for pharmaceutical samples. The importance of titrations in pharmaceutical analysis. If you are in the. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Learn how an electrode works and its. Titration is a common technique used in analytical. Titration In Pharmaceutical Chemistry.
From www.studypool.com
SOLUTION Neutralization titrations strong acid strong base titration Titration In Pharmaceutical Chemistry Learn how an electrode works and its. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of an acid with a standard based. The importance of titrations in pharmaceutical analysis. Titration of a base with a standard acid. If you are in the. • acidimetry • alkalimetry • displacement titration •. Titration In Pharmaceutical Chemistry.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration In Pharmaceutical Chemistry Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration of an acid with a standard based. Titration of a base with a standard acid. If you are in the. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Naoh + hcl → nacl+h2o. Sodium hydroxide with hydrochloric acid. Titration. Titration In Pharmaceutical Chemistry.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration In Pharmaceutical Chemistry If you are in the. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. The importance of titrations in pharmaceutical analysis. Titration of. Titration In Pharmaceutical Chemistry.
From jooinn.com
Free photo Titration School, Medicine, Pharmaceutical Free Titration In Pharmaceutical Chemistry Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. The importance of titrations in pharmaceutical analysis. Learn how an electrode works and its. Titration of a base with a standard acid. Titration of an acid with a standard based. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Understand the difference. Titration In Pharmaceutical Chemistry.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration In Pharmaceutical Chemistry Sodium hydroxide with hydrochloric acid. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of an acid with a standard based. If you are in the. Understand the difference between manual titration and autotitration for pharmaceutical samples. The importance of titrations in pharmaceutical analysis. Titration of a base with a standard. Titration In Pharmaceutical Chemistry.
From www.youtube.com
conductometric titration strong acid vs. strong base YouTube Titration In Pharmaceutical Chemistry Titration of an acid with a standard based. Learn how an electrode works and its. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of a base with a standard acid. • acidimetry • alkalimetry • displacement titration • nonaqueous. Titration In Pharmaceutical Chemistry.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. If you are in the. Titration of an acid with a standard based. Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration of a base with a standard acid. Sodium hydroxide with hydrochloric acid. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown. Titration In Pharmaceutical Chemistry.
From quizizz.com
Titrations Chemistry Quiz Quizizz Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. Naoh + hcl → nacl+h2o. Titration of a base with a standard acid. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Sodium hydroxide with hydrochloric acid. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. If you are in the. Titration of an. Titration In Pharmaceutical Chemistry.
From www.slideshare.net
Precipitation Titration _ Pharmaceutical Analysis _ B. Pharmacy Titration In Pharmaceutical Chemistry Titration of an acid with a standard based. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Sodium hydroxide with hydrochloric acid. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration is a common technique used in analytical chemistry to determine the concentration of an. Titration In Pharmaceutical Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration Titration In Pharmaceutical Chemistry Titration of a base with a standard acid. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Learn how an electrode works and its. Understand the difference between manual titration and autotitration for pharmaceutical samples. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Sodium hydroxide with hydrochloric acid. If. Titration In Pharmaceutical Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Pharmaceutical Chemistry Titration of an acid with a standard based. Sodium hydroxide with hydrochloric acid. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration is a common technique used in analytical chemistry. Titration In Pharmaceutical Chemistry.
From www.studocu.com
Precipitation Titration Notes Pharmaceutical Analysis I Theory Titration In Pharmaceutical Chemistry Learn how an electrode works and its. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Titration of an acid with a standard based. Sodium hydroxide with hydrochloric acid. If you are in the.. Titration In Pharmaceutical Chemistry.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration In Pharmaceutical Chemistry Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of a base with a standard acid. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. If you are in the. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Naoh +. Titration In Pharmaceutical Chemistry.
From mungfali.com
Acid Base Titration Procedure Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Learn how an electrode works and its. If you are in the. Titration of a base with a standard acid. Sodium hydroxide with hydrochloric acid. Naoh + hcl → nacl+h2o. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use. Titration In Pharmaceutical Chemistry.
From www.studocu.com
Complexometric Titration Pharmaceutical Analysis Studocu Titration In Pharmaceutical Chemistry Titration of an acid with a standard based. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Sodium hydroxide with hydrochloric acid. Learn how an electrode works and its. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of a base with a standard acid. Naoh + hcl → nacl+h2o.. Titration In Pharmaceutical Chemistry.
From mungfali.com
Acid Base Titration Calculation Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. Titration of a base with a standard acid. Titration of an acid with a standard based. Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Learn how an electrode works and its. Chemists. Titration In Pharmaceutical Chemistry.
From www.youtube.com
Leveling effect of Solvents Non Aqueous titration Pharmaceutical Titration In Pharmaceutical Chemistry Naoh + hcl → nacl+h2o. The importance of titrations in pharmaceutical analysis. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Sodium hydroxide with hydrochloric acid. Titration of a base with a standard acid. Learn how an electrode works and its. If you are in the. Understand the difference between manual. Titration In Pharmaceutical Chemistry.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration In Pharmaceutical Chemistry Learn how an electrode works and its. The importance of titrations in pharmaceutical analysis. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Sodium hydroxide with hydrochloric acid. If you are in the. Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration of a base with a standard acid.. Titration In Pharmaceutical Chemistry.
From mungfali.com
Acid Base Titration Experiment Titration In Pharmaceutical Chemistry Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Sodium hydroxide with hydrochloric acid. Titration of an acid with a standard based. Learn how an electrode works and its. If you are in the.. Titration In Pharmaceutical Chemistry.
From studiousguy.com
Titration Uses in Real Life StudiousGuy Titration In Pharmaceutical Chemistry Naoh + hcl → nacl+h2o. Understand the difference between manual titration and autotitration for pharmaceutical samples. If you are in the. Sodium hydroxide with hydrochloric acid. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. The importance of titrations in pharmaceutical. Titration In Pharmaceutical Chemistry.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Titration In Pharmaceutical Chemistry If you are in the. Sodium hydroxide with hydrochloric acid. Understand the difference between manual titration and autotitration for pharmaceutical samples. Naoh + hcl → nacl+h2o. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. The importance of titrations in pharmaceutical. Titration In Pharmaceutical Chemistry.
From www.visionlearning.com
Acids and Bases I Scientists and Research Visionlearning Titration In Pharmaceutical Chemistry Learn how an electrode works and its. Titration of an acid with a standard based. The importance of titrations in pharmaceutical analysis. Sodium hydroxide with hydrochloric acid. If you are in the. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration of a base with a standard acid. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations,. Titration In Pharmaceutical Chemistry.
From www.studypool.com
SOLUTION Neutralization titrations strong acid strong base titration Titration In Pharmaceutical Chemistry Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. The importance of titrations in pharmaceutical analysis. Naoh + hcl → nacl+h2o. Learn how an electrode works and its. Titration of an acid with a. Titration In Pharmaceutical Chemistry.
From mungfali.com
Titration Steps Titration In Pharmaceutical Chemistry Sodium hydroxide with hydrochloric acid. Titration of a base with a standard acid. The importance of titrations in pharmaceutical analysis. Learn how an electrode works and its. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use indirect redox titrations, often referred to as back titrations, as. Titration In Pharmaceutical Chemistry.
From www.alamy.com
Titration hires stock photography and images Alamy Titration In Pharmaceutical Chemistry Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of a base with a standard acid. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Sodium hydroxide with hydrochloric acid. Learn how. Titration In Pharmaceutical Chemistry.
From www.thoughtco.com
Redox Titration Definition (Chemistry) Titration In Pharmaceutical Chemistry • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Sodium hydroxide with hydrochloric acid. Understand the difference between manual titration and autotitration for pharmaceutical samples. The importance of titrations in pharmaceutical analysis. Learn how an electrode works and its. If you are in the. Titration of a base with a standard acid. Titration of an acid with a standard. Titration In Pharmaceutical Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration In Pharmaceutical Chemistry Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Understand the difference between manual titration and autotitration for pharmaceutical samples. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. The importance of titrations in pharmaceutical analysis. If you are in the. • acidimetry. Titration In Pharmaceutical Chemistry.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration In Pharmaceutical Chemistry The importance of titrations in pharmaceutical analysis. Titration of an acid with a standard based. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Understand the difference between manual titration and autotitration for pharmaceutical samples. Titration of a base with a standard acid. Sodium hydroxide with hydrochloric acid. Learn how an electrode works and its. If you are in. Titration In Pharmaceutical Chemistry.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Titration In Pharmaceutical Chemistry Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. Learn how an electrode works and its. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Sodium hydroxide with hydrochloric acid. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical. Titration In Pharmaceutical Chemistry.
From roqed.com
Titration (pH) curves ROQED Titration In Pharmaceutical Chemistry If you are in the. Understand the difference between manual titration and autotitration for pharmaceutical samples. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Learn how an electrode works and its. Sodium hydroxide with hydrochloric acid. Titration is a common technique used in analytical chemistry to. Titration In Pharmaceutical Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration In Pharmaceutical Chemistry If you are in the. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Learn how an electrode works and its. Sodium hydroxide with hydrochloric acid. Understand the difference between manual titration and autotitration for pharmaceutical samples. Naoh + hcl → nacl+h2o. Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in.. Titration In Pharmaceutical Chemistry.
From fyopywglf.blob.core.windows.net
What Is Redox Titration In Pharmaceutical Analysis at Ethel Willingham blog Titration In Pharmaceutical Chemistry Titration of a base with a standard acid. The importance of titrations in pharmaceutical analysis. Learn how an electrode works and its. Titration of an acid with a standard based. Naoh + hcl → nacl+h2o. • acidimetry • alkalimetry • displacement titration • nonaqueous titration. Titration is a common technique used in analytical chemistry to determine the concentration of an. Titration In Pharmaceutical Chemistry.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration In Pharmaceutical Chemistry Chemists use indirect redox titrations, often referred to as back titrations, as a powerful analytical technique in. Titration of a base with a standard acid. Sodium hydroxide with hydrochloric acid. Titration of an acid with a standard based. Titration is a common technique used in analytical chemistry to determine the concentration of an unknown solution by. • acidimetry • alkalimetry. Titration In Pharmaceutical Chemistry.