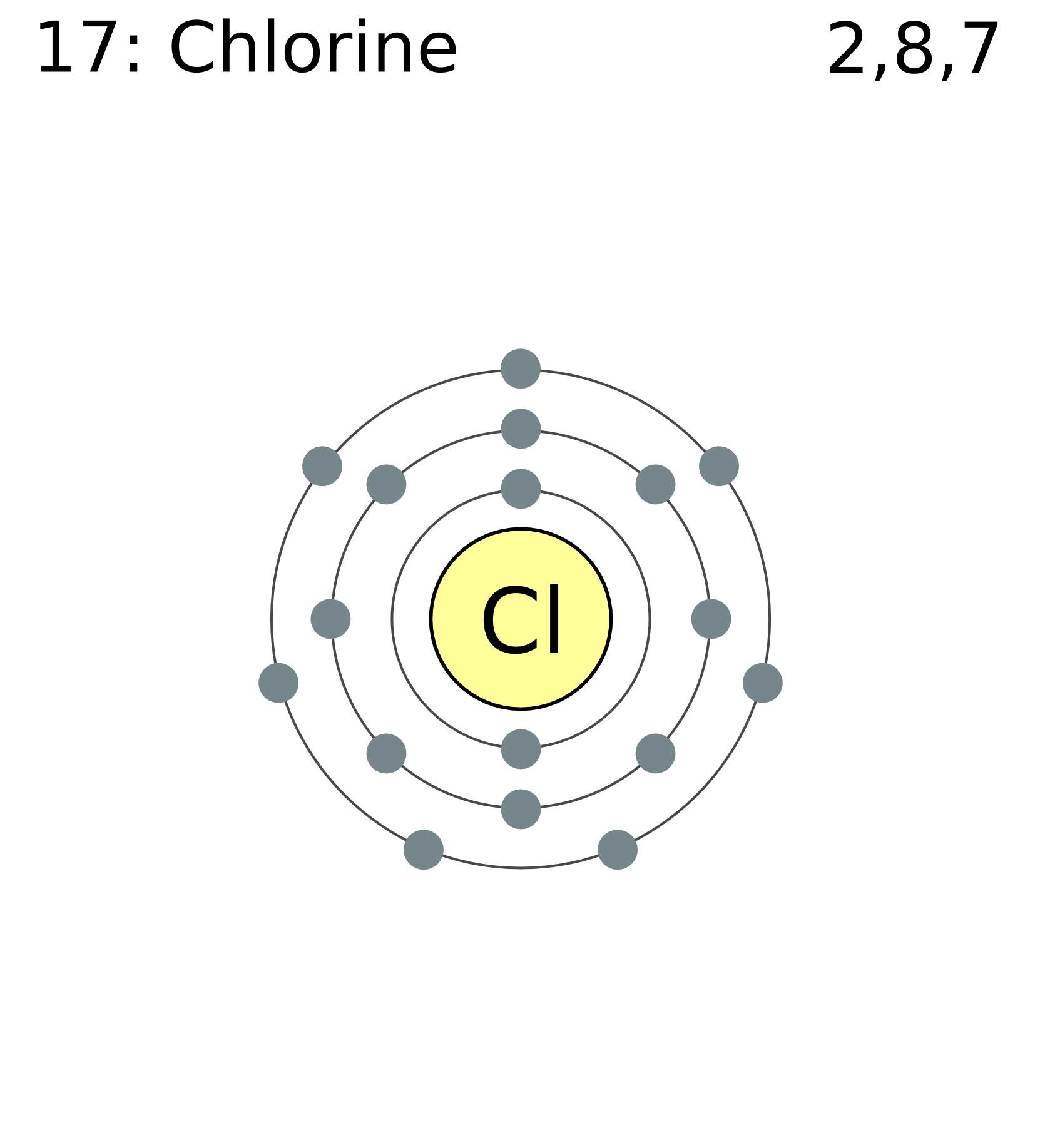
Chlorine Electrons . Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Find out its physical, chemical, biological and historical. Chlorine has 17 protons and 17 electrons in its neutral atom. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. It has the electron configuration [ne] 3s 2 3p 5 and is. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. On an industrial scale, chlorine is produced by. See the table of sublevels, orbitals and. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how to arrange electrons in an atom using electron configurations, which are unique to each element.
from commons.wikimedia.org
Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Chlorine has 17 protons and 17 electrons in its neutral atom. See the table of sublevels, orbitals and. On an industrial scale, chlorine is produced by. It has the electron configuration [ne] 3s 2 3p 5 and is. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17.
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine Electrons Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. On an industrial scale, chlorine is produced by. Chlorine has 17 protons and 17 electrons in its neutral atom. Find out its physical, chemical, biological and historical. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. See the table of sublevels, orbitals and. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. It has the electron configuration [ne] 3s 2 3p 5 and is.
From
Chlorine Electrons Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Find out its physical, chemical, biological and historical. Find out the rules and patterns for filling subshells and the maximum number of electrons in each. Chlorine Electrons.
From
Chlorine Electrons Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Find out its physical, chemical, biological and historical. See the. Chlorine Electrons.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine Electrons Chlorine has 17 protons and 17 electrons in its neutral atom. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. See the table of sublevels, orbitals and. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn. Chlorine Electrons.
From
Chlorine Electrons On an industrial scale, chlorine is produced by. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Find out the rules and patterns for filling subshells and the maximum number of electrons. Chlorine Electrons.
From
Chlorine Electrons Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. See the table of sublevels, orbitals and. Learn how to write. Chlorine Electrons.
From
Chlorine Electrons Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. On an industrial scale, chlorine is produced by. See the table of sublevels, orbitals and. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Find out its physical, chemical, biological and. Chlorine Electrons.
From
Chlorine Electrons It has the electron configuration [ne] 3s 2 3p 5 and is. See the table of sublevels, orbitals and. On an industrial scale, chlorine is produced by. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Chlorine. Chlorine Electrons.
From
Chlorine Electrons Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn about chlorine, a greenish yellow. Chlorine Electrons.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Electrons On an industrial scale, chlorine is produced by. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Find out its physical, chemical, biological and historical. See the table of sublevels, orbitals and. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element.. Chlorine Electrons.
From
Chlorine Electrons Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Find out the rules and. Chlorine Electrons.
From
Chlorine Electrons Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. It has the electron configuration [ne] 3s 2 3p 5 and is. See the table of sublevels, orbitals and. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Chlorine has 17 protons. Chlorine Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electrons On an industrial scale, chlorine is produced by. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal. Chlorine Electrons.
From
Chlorine Electrons See the table of sublevels, orbitals and. Find out its physical, chemical, biological and historical. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Small amounts of chlorine. Chlorine Electrons.
From
Chlorine Electrons See the table of sublevels, orbitals and. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. It has the electron configuration [ne] 3s 2 3p 5 and is. Learn how electrons are grouped into shells and. Chlorine Electrons.
From
Chlorine Electrons Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Find out its physical, chemical, biological and historical. Find out the rules and patterns for filling subshells and the. Chlorine Electrons.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Electrons Find out its physical, chemical, biological and historical. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. On an industrial scale, chlorine is produced by. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn how to arrange electrons. Chlorine Electrons.
From
Chlorine Electrons Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Find out its physical, chemical, biological and historical. See the table of sublevels, orbitals and. It has the electron configuration [ne] 3s. Chlorine Electrons.
From
Chlorine Electrons Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn about. Chlorine Electrons.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Electrons Chlorine has 17 protons and 17 electrons in its neutral atom. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Find out its physical, chemical, biological and historical. On an industrial scale, chlorine is produced by. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic. Chlorine Electrons.
From
Chlorine Electrons Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. On an industrial scale, chlorine. Chlorine Electrons.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electrons Find out its physical, chemical, biological and historical. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. It has the electron configuration [ne] 3s 2 3p 5 and is. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). On an industrial scale, chlorine. Chlorine Electrons.
From
Chlorine Electrons On an industrial scale, chlorine is produced by. Chlorine has 17 protons and 17 electrons in its neutral atom. It has the electron configuration [ne] 3s 2 3p 5 and is. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Learn about chlorine, a greenish yellow gas with 17 electrons and. Chlorine Electrons.
From
Chlorine Electrons Find out its physical, chemical, biological and historical. Chlorine has 17 protons and 17 electrons in its neutral atom. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how to arrange. Chlorine Electrons.
From
Chlorine Electrons It has the electron configuration [ne] 3s 2 3p 5 and is. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. On an industrial scale, chlorine is produced by. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). See the table. Chlorine Electrons.
From
Chlorine Electrons Chlorine has 17 protons and 17 electrons in its neutral atom. It has the electron configuration [ne] 3s 2 3p 5 and is. On an industrial scale, chlorine is produced by. Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Learn how to arrange electrons in an atom using electron configurations,. Chlorine Electrons.
From
Chlorine Electrons Find out the rules and patterns for filling subshells and the maximum number of electrons in each subshell. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. On an industrial scale, chlorine is produced by. Learn how electrons are grouped into shells and subshells according to their energies and how to write. Chlorine Electrons.
From
Chlorine Electrons Chlorine has 17 protons and 17 electrons in its neutral atom. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Find out its physical, chemical, biological and historical. On an industrial scale, chlorine is produced by. Learn how to arrange electrons in an atom using electron configurations, which are unique to each. Chlorine Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Electrons On an industrial scale, chlorine is produced by. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Chlorine has 17 protons and 17 electrons in its neutral atom. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how electrons are grouped into shells and subshells. Chlorine Electrons.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Electrons Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. Chlorine has 17 protons and 17 electrons in its neutral atom. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Find out its physical, chemical, biological and historical. Learn how electrons are. Chlorine Electrons.
From
Chlorine Electrons Chlorine has 17 protons and 17 electrons in its neutral atom. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). It has the electron configuration [ne] 3s 2 3p 5 and is. Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17.. Chlorine Electrons.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Electrons Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). Learn how to write the full and abbreviated ground state electron. Chlorine Electrons.
From
Chlorine Electrons Learn how electrons are grouped into shells and subshells according to their energies and how to write electron configurations for atoms. Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Find out its physical, chemical, biological and historical. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure. Chlorine Electrons.
From
Chlorine Electrons Learn how to arrange electrons in an atom using electron configurations, which are unique to each element. Small amounts of chlorine can be produced in the lab by oxidizing \ (hcl\) with \ (mno_2\). It has the electron configuration [ne] 3s 2 3p 5 and is. Find out its physical, chemical, biological and historical. Learn how to write the full. Chlorine Electrons.
From
Chlorine Electrons It has the electron configuration [ne] 3s 2 3p 5 and is. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Find out its physical, chemical, biological and historical. On an industrial scale, chlorine is produced by. Chlorine has 17 protons and 17 electrons in its neutral atom. Learn how to write the full. Chlorine Electrons.
From
Chlorine Electrons Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. It has the electron configuration [ne] 3s 2 3p 5 and is. Learn about chlorine, a greenish yellow gas with 17 electrons and the shell structure 2.8.7. Find out its physical, chemical, biological and historical. Find out the rules and. Chlorine Electrons.