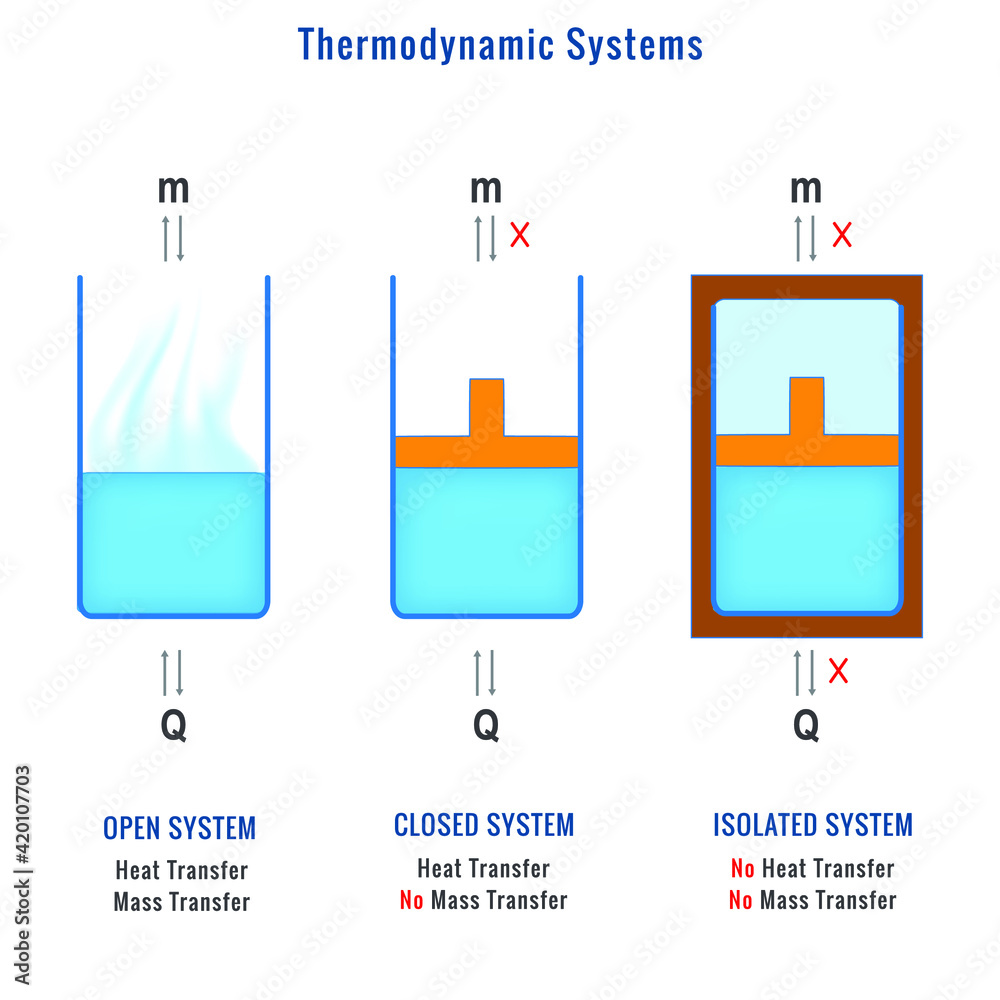
Isolated Vs Open System . Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. Open systems allow energy and matter (stuff) to enter and leave the system. (1) open system, (2) closed system and (3) isolated system. This physics video tutorial provides a basic introduction into open systems, closed systems,. These are the types of thermodynamic system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open, closed, and isolated systems. Let me tell you some basics before starting this.
from stock.adobe.com
This physics video tutorial provides a basic introduction into open systems, closed systems,. (1) open system, (2) closed system and (3) isolated system. Let me tell you some basics before starting this. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. These are the types of thermodynamic system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open systems allow energy and matter (stuff) to enter and leave the system. Open, closed, and isolated systems. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their.
Vecteur Stock Different types of Thermodynamic Systems, Open System
Isolated Vs Open System Open systems allow energy and matter (stuff) to enter and leave the system. Open systems allow energy and matter (stuff) to enter and leave the system. These are the types of thermodynamic system. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. This physics video tutorial provides a basic introduction into open systems, closed systems,. (1) open system, (2) closed system and (3) isolated system. Let me tell you some basics before starting this. Open, closed, and isolated systems.
From pharmacydocs.blogspot.com
Essential Pharma Documents 1205 Thermodynamics Isolated Vs Open System A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. These are the types of thermodynamic system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff). Isolated Vs Open System.
From www.youtube.com
Types of System in Thermodynamics. Types of Thermodynamics System. open Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Let me tell you some basics before starting this. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and. Isolated Vs Open System.
From in.pinterest.com
an open closed, isolated system is shown in this slide Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. These are the types of thermodynamic system. A pan on the stove is an open system because water can evaporate or be. Isolated Vs Open System.
From www.alamy.com
Different types of Thermodynamic Systems, Open System, Closed System Isolated Vs Open System Open, closed, and isolated systems. This physics video tutorial provides a basic introduction into open systems, closed systems,. Let me tell you some basics before starting this. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. A pan on the stove is an open. Isolated Vs Open System.
From www.differencebetween.com
Difference Between Isolated System and Closed System Compare the Isolated Vs Open System A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. These are the types of thermodynamic system. Open systems allow energy and matter (stuff) to enter and leave the system. (1) open system, (2) closed system. Isolated Vs Open System.
From www.slideserve.com
PPT Open, Closed and Isolated Systems PowerPoint Presentation, free Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open, closed, and isolated systems. Open systems allow energy and matter (stuff) to enter and leave the system. Let me tell you some basics before starting this. A closed system is a system that does not interact with its. Isolated Vs Open System.
From pediaa.com
Difference Between Open and Closed System Definition, Characteristics Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open, closed, and isolated systems. Open systems can exchange both matter and energy with their surroundings,. Isolated Vs Open System.
From quizlet.com
Compare and contrast an open system and a closed system. Use Quizlet Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. Let me tell you some basics before starting this. These are the types of thermodynamic system. Open systems allow energy and matter (stuff) to enter and leave the system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not. Isolated Vs Open System.
From slideplayer.com
Chemical Reaction Networks ppt download Isolated Vs Open System Open systems allow energy and matter (stuff) to enter and leave the system. Let me tell you some basics before starting this. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. (1) open system, (2). Isolated Vs Open System.
From www.youtube.com
Thermodynamic tricks, open, closed & isolated system, first law YouTube Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. (1) open system, (2) closed. Isolated Vs Open System.
From www.youtube.com
Earth as an Isolated and Open systems YouTube Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. Let me tell you some basics before starting this. (1) open system, (2) closed system and (3) isolated system. Open, closed, and isolated systems. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan. Isolated Vs Open System.
From brainly.in
Discuss closed, open and isolated thermodynamic system with neat sketch Isolated Vs Open System Open systems allow energy and matter (stuff) to enter and leave the system. Let me tell you some basics before starting this. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. A closed system is. Isolated Vs Open System.
From lawofthermodynamicsinfo.com
What Is Thermodynamic System? Open, Closed & Isolated (With Examples) Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. These are the types of thermodynamic system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff) to enter and leave the system. A closed system is a system. Isolated Vs Open System.
From www.slideshare.net
Thermodynamics Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. Open, closed, and isolated systems. This physics video tutorial provides a basic introduction into open systems, closed systems,. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff) to enter and leave. Isolated Vs Open System.
From surfguppy.com
Themodynamic Systems Open, Closed & Isolated Systems Isolated Vs Open System Open, closed, and isolated systems. Open systems allow energy and matter (stuff) to enter and leave the system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. This physics video tutorial provides a basic introduction into open systems, closed systems,. These are the types of thermodynamic system. Let. Isolated Vs Open System.
From www.slideserve.com
PPT Open, Closed and Isolated Systems PowerPoint Presentation, free Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. Let me tell you some basics before starting this. A pan on the stove is an open system because water can evaporate or be poured. Isolated Vs Open System.
From www.youtube.com
What are Open, Closed and Isolated Systems? Understand the concept with Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. (1) open system, (2) closed system and (3) isolated system. Open systems allow energy and matter (stuff) to enter and leave the system. Let me tell you some basics before starting this. These are the types of thermodynamic system. A closed system is a system that does. Isolated Vs Open System.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation ID2654677 Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. These are the types of thermodynamic system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the. Isolated Vs Open System.
From www.slideserve.com
PPT OPEN AND CLOSED SYSTEMS PowerPoint Presentation, free download Isolated Vs Open System A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open systems allow energy and matter (stuff) to enter and leave the system. (1) open system, (2) closed system and (3) isolated system. This physics video. Isolated Vs Open System.
From www.slideserve.com
PPT Classical Thermodynamics A Portion of a Lecture Borrowed from Isolated Vs Open System These are the types of thermodynamic system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open systems allow energy and matter (stuff) to enter and leave the system. Let me tell you some basics. Isolated Vs Open System.
From mechanicalinventions.blogspot.com
What is system, boundary and surrounding is thermodynamics Isolated Vs Open System These are the types of thermodynamic system. This physics video tutorial provides a basic introduction into open systems, closed systems,. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. A pan on the stove is an open system because water can evaporate or be poured in, and heat. Isolated Vs Open System.
From studiousguy.com
3 Isolated System Examples in Real Life StudiousGuy Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff) to enter and leave the system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove. Isolated Vs Open System.
From www.youtube.com
Open, CLosed & Isolated Systems YouTube Isolated Vs Open System Open, closed, and isolated systems. This physics video tutorial provides a basic introduction into open systems, closed systems,. Let me tell you some basics before starting this. These are the types of thermodynamic system. Open systems allow energy and matter (stuff) to enter and leave the system. A pan on the stove is an open system because water can evaporate. Isolated Vs Open System.
From pediaa.com
What is the Difference Between Isolated System and Closed System Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open, closed, and isolated systems. Let me tell you some basics before starting this. Open systems allow energy and matter (stuff) to enter and leave the system. (1) open system, (2) closed system and (3) isolated system. This physics. Isolated Vs Open System.
From stock.adobe.com
Vecteur Stock Different types of Thermodynamic Systems, Open System Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. These are the types of thermodynamic system. Let me tell you some basics before starting this. Open systems allow energy and matter (stuff) to enter and leave the system.. Isolated Vs Open System.
From dizz.com
Thermodynamics systemClosed, Open, Isolated system with example, pdf Isolated Vs Open System A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. Open, closed, and isolated systems. This physics video tutorial provides a basic introduction into open systems, closed systems,. Open systems allow energy and matter (stuff) to. Isolated Vs Open System.
From www.biologyonline.com
System Definition and Examples Biology Online Dictionary Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff) to enter and leave the system. These are the types of thermodynamic system. A closed system is a system that does not interact with its environment, meaning that no matter or energy. Isolated Vs Open System.
From www.youtube.com
Types of systems (Isolated, Closed, Open) YouTube Isolated Vs Open System A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. These are the types of thermodynamic system. Open, closed, and isolated systems. Let me tell you some basics before starting this. This physics video tutorial provides a basic introduction into open systems, closed systems,. Open. Isolated Vs Open System.
From www.youtube.com
Open System, Closed System and Isolated System Thermodynamics Isolated Vs Open System Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Open systems allow energy and matter (stuff) to enter and leave the system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove. Isolated Vs Open System.
From classnotes.org.in
Basic Terms And Concepts In Thermodynamics Chemistry, Class 11 Isolated Vs Open System This physics video tutorial provides a basic introduction into open systems, closed systems,. Let me tell you some basics before starting this. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. (1) open system, (2). Isolated Vs Open System.
From www.differencebetween.com
Difference Between Closed System and Open System Compare the Isolated Vs Open System A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. Open, closed, and isolated systems. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy but not matter with their. Let me tell you some basics before starting this.. Isolated Vs Open System.
From pediaa.com
Difference Between Adiabatic and Isolated System Definition Isolated Vs Open System A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. (1) open system, (2) closed system and (3) isolated system. Open, closed, and isolated systems. A pan on the stove is an open system because water can evaporate or be poured in, and heat can. Isolated Vs Open System.
From www.slideserve.com
PPT Classical Thermodynamics A Portion of a Lecture Borrowed from Isolated Vs Open System A closed system is a system that does not interact with its environment, meaning that no matter or energy can enter or leave the system. Open, closed, and isolated systems. These are the types of thermodynamic system. Let me tell you some basics before starting this. This physics video tutorial provides a basic introduction into open systems, closed systems,. Open. Isolated Vs Open System.
From www.difference.minaprem.com
Difference Between Open System, Closed System and Isolated System Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. Open, closed, and isolated systems. Open systems allow energy and matter (stuff) to enter and leave the system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the. Isolated Vs Open System.
From present5.com
Chemical thermodynamics lesson plan 1 Types of Isolated Vs Open System (1) open system, (2) closed system and (3) isolated system. A pan on the stove is an open system because water can evaporate or be poured in, and heat can enter the pan if the stove is turned on, and leave the pan also. A closed system is a system that does not interact with its environment, meaning that no. Isolated Vs Open System.