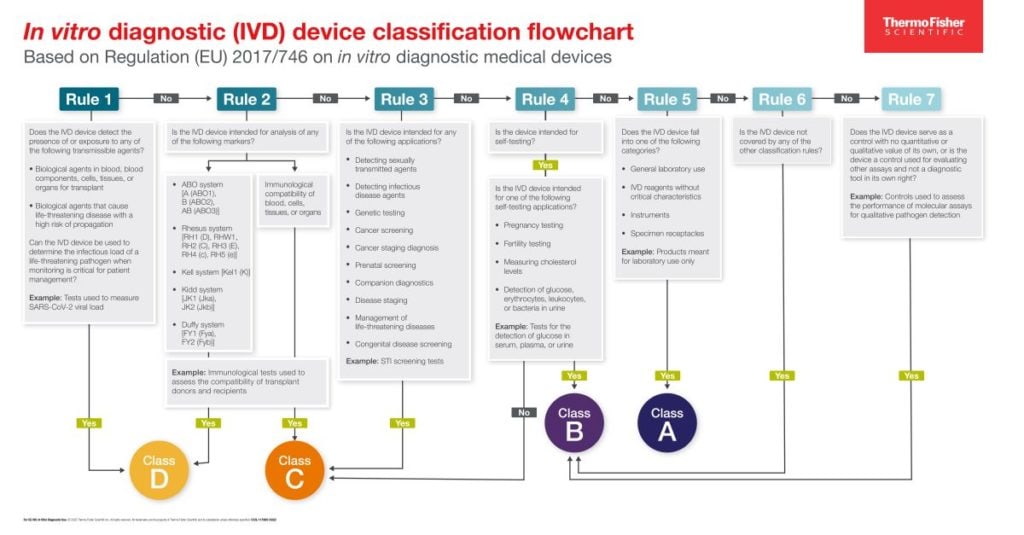
Classification Of Ivd Medical Devices . the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance document is one of a series that together describe a global regulatory model for medical devices. Guidance on classification rules for ivd.
from www.thermofisher.com
this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. Guidance on classification rules for ivd.
IVDD vs. IVDR Classifications Defined and Compared OEMpowered
Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance document is one of a series that together describe a global regulatory model for medical devices. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr).
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Classification Of Ivd Medical Devices the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb). Classification Of Ivd Medical Devices.
From rs-ness.com
IVD Classification Under IVDR Definition RS NESS Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this guidance document is one of a series that together describe a global regulatory model for medical devices. . Classification Of Ivd Medical Devices.
From dokumen.tips
(PDF) General Classification System for IVD Medical Devicesmdb.gov.my Classification Of Ivd Medical Devices the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd). Classification Of Ivd Medical Devices.
From www.artfulcompliance.com
00014 REV01 IVDR IVD Classification Classification Of Ivd Medical Devices Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this guidance document is one of a series that together describe a global regulatory model for medical. Classification Of Ivd Medical Devices.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Classification Of Ivd Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member. Classification Of Ivd Medical Devices.
From gsap.co.il
In Vitro Diagnostic Medical Device (IVD) in the EU Gsap Classification Of Ivd Medical Devices the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in. Classification Of Ivd Medical Devices.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this guidance document is one of a series that together describe a global regulatory model for medical devices. Guidance on classification rules for. Classification Of Ivd Medical Devices.
From www.scribd.com
Classification and Registration Pathways of Medical Devices and IVDs in Classification Of Ivd Medical Devices Guidance on classification rules for ivd. this guidance document is one of a series that together describe a global regulatory model for medical devices. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. the 4th indent of the definition of a medical device specifies “providing. Classification Of Ivd Medical Devices.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro. Classification Of Ivd Medical Devices.
From exoelsutj.blob.core.windows.net
Classification Of Ivd Medical Devices In Europe at Eugene Lee blog Classification Of Ivd Medical Devices this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination. Classification Of Ivd Medical Devices.
From www.qualitymeddev.com
The New IVDR Classification for InVitro Diagnostic Devices Classification Of Ivd Medical Devices this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Guidance on classification rules for ivd. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to. Classification Of Ivd Medical Devices.
From medicaldevices.freyrsolutions.com
IVD Classification under the EU IVDR 2017/746 Regulations Freyr Classification Of Ivd Medical Devices Guidance on classification rules for ivd. this guidance document is one of a series that together describe a global regulatory model for medical devices. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers. Classification Of Ivd Medical Devices.
From www.cognidox.com
New IVD regulation is coming. are you ready? Classification Of Ivd Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. . Classification Of Ivd Medical Devices.
From www.researchgate.net
Medical device types reported in the submitted MIR pilot forms. IVD in Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination. Classification Of Ivd Medical Devices.
From www.scilife.io
In Vitro Diagnostics (IVD) A Complete Overview Scilife Classification Of Ivd Medical Devices the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. this guidance document is one of a series that together describe a. Classification Of Ivd Medical Devices.
From mdrc-consulting.com
Europe's IVD regulatory approval process MDRC Classification Of Ivd Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical. Classification Of Ivd Medical Devices.
From www.scribd.com
2 Classification of IVD Medical Devices Therapeutic Goods Classification Of Ivd Medical Devices this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are. Classification Of Ivd Medical Devices.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Classification Of Ivd Medical Devices this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance document is one of a series that together describe a global regulatory model for medical devices. the fda classifies medical. Classification Of Ivd Medical Devices.
From www.tga.gov.au
Including IVD medical devices in the ARTG Therapeutic Goods Classification Of Ivd Medical Devices Guidance on classification rules for ivd. this guidance document is one of a series that together describe a global regulatory model for medical devices. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance, relating to the application of regulation (eu) 2017/746 on in. Classification Of Ivd Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this guidance document is one of a series that together describe a global regulatory model for medical devices. Guidance on classification rules for. Classification Of Ivd Medical Devices.
From clariscience.com
Classification of medical devices and IVDs Clariscience Classification Of Ivd Medical Devices Guidance on classification rules for ivd. this guidance document is one of a series that together describe a global regulatory model for medical devices. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers. Classification Of Ivd Medical Devices.
From accuprecnews.wixsite.com
CDSCO releases Risk classification of newly notified Medical Devices Classification Of Ivd Medical Devices Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the. Classification Of Ivd Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Classification Of Ivd Medical Devices Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to. Classification Of Ivd Medical Devices.
From www.pdffiller.com
Fillable Online tga gov Classification of IVD medical devices Medical Classification Of Ivd Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. Guidance. Classification Of Ivd Medical Devices.
From exoelsutj.blob.core.windows.net
Classification Of Ivd Medical Devices In Europe at Eugene Lee blog Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Guidance. Classification Of Ivd Medical Devices.
From cliniexperts.com
CDSCO Recently Classified 80 InVitro Diagnostic Medical Devices For Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. Guidance on classification rules for ivd. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the 4th indent of the definition of a medical device specifies “providing information. Classification Of Ivd Medical Devices.
From www.thermofisher.com
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Classification Of Ivd Medical Devices the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to in vitro diagnostics (ivds), which are a subgroup of medical devices. this guidance document is one of a series that together describe a. Classification Of Ivd Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the. Classification Of Ivd Medical Devices.
From exoelsutj.blob.core.windows.net
Classification Of Ivd Medical Devices In Europe at Eugene Lee blog Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the. Classification Of Ivd Medical Devices.
From vyomusconsulting.com
MedicalDevice & IVD Registration Process Overview Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. Guidance on classification rules for ivd. this guidance document is one of a series that together describe a global regulatory model for medical devices. the 4th indent of the definition of a medical device specifies “providing information by means of. Classification Of Ivd Medical Devices.
From rs-ness.com
IVD Classification Under IVDR Definition RS NESS Classification Of Ivd Medical Devices Guidance on classification rules for ivd. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx. Classification Of Ivd Medical Devices.
From dokumen.tips
(PDF) Principles of In Vitro Diagnostic (IVD) Medical Devices Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination. Classification Of Ivd Medical Devices.
From vdocuments.mx
General Classification System for IVD Medical Devicesmdb.gov.my/mdb Classification Of Ivd Medical Devices Guidance on classification rules for ivd. principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro examination of specimens derived from the human body […]” as a medical purpose and thus refers to. Classification Of Ivd Medical Devices.
From fyocylyim.blob.core.windows.net
What Is A Class Iv Medical Device at Arron Cooper blog Classification Of Ivd Medical Devices the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. this guidance document is one of a series that together describe a global regulatory model for medical devices. the 4th indent of the definition of a medical device specifies “providing information by means of in vitro. Classification Of Ivd Medical Devices.
From www.youtube.com
Parameters for Classification of IVD Medical Devices Medical Devices Classification Of Ivd Medical Devices principles of in vitro diagnostic (ivd) medical devices classification pdf (212.61 kb) docx (119.58 kb) member sites. the fda classifies medical devices, including ivd products, into class i, ii, or iii according to the level of regulatory control. Guidance on classification rules for ivd. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro. Classification Of Ivd Medical Devices.