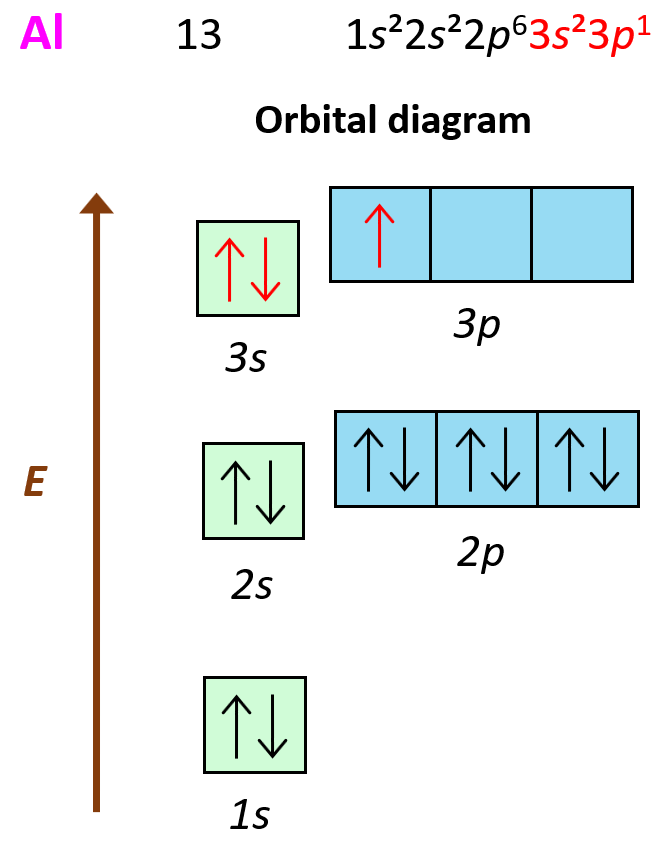
Electron Removal Order . A cation (positively charged ion) forms when one or more electrons are removed from a. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; For main group elements, the electrons that were added last are the first electrons removed. This means that electrons will be removed from the 4s subshell before any electrons are. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. The electrons will be removed from the highest energy shell in the neutral configuration. Ions are formed when atoms gain or lose electrons. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. For transition metals and inner transition metals, however, electrons in the s orbital are. A cation (positively charged ion) forms when one or more electrons are removed. Also, hund's rule still applies. We have seen that ions are formed when atoms gain or lose electrons.
from general.chemistrysteps.com
This means that electrons will be removed from the 4s subshell before any electrons are. We have seen that ions are formed when atoms gain or lose electrons. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; For transition metals and inner transition metals, however, electrons in the s orbital are. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. Also, hund's rule still applies. A cation (positively charged ion) forms when one or more electrons are removed from a. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. A cation (positively charged ion) forms when one or more electrons are removed. For main group elements, the electrons that were added last are the first electrons removed.
Ionization energy Chemistry Steps
Electron Removal Order Also, hund's rule still applies. For transition metals and inner transition metals, however, electrons in the s orbital are. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; This means that electrons will be removed from the 4s subshell before any electrons are. A cation (positively charged ion) forms when one or more electrons are removed. A cation (positively charged ion) forms when one or more electrons are removed from a. For main group elements, the electrons that were added last are the first electrons removed. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. Also, hund's rule still applies. Ions are formed when atoms gain or lose electrons. We have seen that ions are formed when atoms gain or lose electrons. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. The electrons will be removed from the highest energy shell in the neutral configuration.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Electron Removal Order Electrons are typically removed from the valence shells, which are the highest s and p orbitals. A cation (positively charged ion) forms when one or more electrons are removed from a. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a. Electron Removal Order.
From neetlab.com
Ionization Enthalpy NEET Lab Electron Removal Order This means that electrons will be removed from the 4s subshell before any electrons are. For main group elements, the electrons that were added last are the first electrons removed. We have seen that ions are formed when atoms gain or lose electrons. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. A. Electron Removal Order.
From www.pinterest.com
Ionization Energy the amount of energy required to remove an electron Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed. Also, hund's rule still applies. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. The electrons will be removed from the highest energy shell in the neutral configuration. Electrons are typically. Electron Removal Order.
From www.youtube.com
Molecular Orbital (MO) Diagram for O2(2) YouTube Electron Removal Order This means that electrons will be removed from the 4s subshell before any electrons are. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. For main group elements, the electrons that were added last are the first electrons removed. Also, hund's rule still applies. We have seen that ions are formed when atoms. Electron Removal Order.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Electron Removal Order For main group elements, the electrons that were added last are the first electrons removed. Also, hund's rule still applies. A cation (positively charged ion) forms when one or more electrons are removed from a. We have seen that ions are formed when atoms gain or lose electrons. This means that electrons will be removed from the 4s subshell before. Electron Removal Order.
From www.breakingatom.com
Electron Affinity of The Elements Electron Removal Order We have seen that ions are formed when atoms gain or lose electrons. The electrons will be removed from the highest energy shell in the neutral configuration. For transition metals and inner transition metals, however, electrons in the s orbital are. A cation (positively charged ion) forms when one or more electrons are removed from a. This means that electrons. Electron Removal Order.
From www.chegg.com
Solved VISUALIZATION Drawing Lewis Structures Clear Electron Removal Order These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. Ions are formed when atoms gain or lose electrons. We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed. Electron Removal Order.
From www.britannica.com
Periodic table Elements, Groups, Properties Britannica Electron Removal Order We have seen that ions are formed when atoms gain or lose electrons. For main group elements, the electrons that were added last are the first electrons removed. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Ions. Electron Removal Order.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Removal Order Ions are formed when atoms gain or lose electrons. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. A cation (positively charged ion) forms when one or more electrons are removed from a. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the. Electron Removal Order.
From www.youtube.com
Electron shielding YouTube Electron Removal Order Electrons are typically removed from the valence shells, which are the highest s and p orbitals. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Ions are formed when atoms gain or lose electrons. A cation (positively charged. Electron Removal Order.
From www.youtube.com
Adding and Removing Electrons, Two Minute Tuesday AQA A Level Electron Removal Order The electrons will be removed from the highest energy shell in the neutral configuration. For main group elements, the electrons that were added last are the first electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are. A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are. Electron Removal Order.
From gzscienceclassonline.weebly.com
1. Electron Configuration Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed. For transition metals and inner transition metals, however, electrons in the s orbital are. Also, hund's rule still applies. We have seen that ions are formed when atoms gain or lose electrons. For main group elements, the electrons that were added last are the first electrons removed.. Electron Removal Order.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Removal Order These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. A cation (positively charged ion) forms when one or more electrons are removed. The electrons will be removed from the highest energy shell in the neutral configuration. For main group elements, the electrons that were. Electron Removal Order.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube Electron Removal Order Also, hund's rule still applies. Ions are formed when atoms gain or lose electrons. For main group elements, the electrons that were added last are the first electrons removed. The electrons will be removed from the highest energy shell in the neutral configuration. A cation (positively charged ion) forms when one or more electrons are removed from a. The electron. Electron Removal Order.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed from a. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; The electrons will be removed from the highest energy shell in the neutral configuration.. Electron Removal Order.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Electron Removal Order The electrons will be removed from the highest energy shell in the neutral configuration. A cation (positively charged ion) forms when one or more electrons are removed. For main group elements, the electrons that were added last are the first electrons removed. A cation (positively charged ion) forms when one or more electrons are removed from a. This means that. Electron Removal Order.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Electron Removal Order Electrons are typically removed from the valence shells, which are the highest s and p orbitals. For main group elements, the electrons that were added last are the first electrons removed. Also, hund's rule still applies. A cation (positively charged ion) forms when one or more electrons are removed from a. The electron removed during the ionization of beryllium ([he]2s. Electron Removal Order.
From www.sciencecoverage.com
How Many Valence Electrons Does Hydrogen (H) Have? [Valency of H & H+] Electron Removal Order Ions are formed when atoms gain or lose electrons. For main group elements, the electrons that were added last are the first electrons removed. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; The electrons will be removed. Electron Removal Order.
From abbiebolton.z21.web.core.windows.net
Successive Ionization Energies Chart Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed from a. A cation (positively charged ion) forms when one or more electrons are removed. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. Also, hund's rule still applies. For transition. Electron Removal Order.
From chemistry291.blogspot.com
【Tips】Electron Configuration(with Video)How Do You Get the Electron Electron Removal Order Also, hund's rule still applies. We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed. This means that electrons will be removed from the 4s subshell before any electrons are. Electrons are typically removed from the valence shells, which are the highest s and. Electron Removal Order.
From www.researchgate.net
Bond order of diatomic species having (120) electrons Download Table Electron Removal Order The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; For transition metals and inner transition metals, however, electrons in the s orbital are. Electrons are typically removed from the valence shells, which are the highest s and p. Electron Removal Order.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Electron Removal Order The electrons will be removed from the highest energy shell in the neutral configuration. A cation (positively charged ion) forms when one or more electrons are removed. This means that electrons will be removed from the 4s subshell before any electrons are. These are the electrons in the highest energy level, and so it is logical that they will be. Electron Removal Order.
From www.chegg.com
Solved The ionization energy of an atom is the energy Electron Removal Order Ions are formed when atoms gain or lose electrons. This means that electrons will be removed from the 4s subshell before any electrons are. For main group elements, the electrons that were added last are the first electrons removed. We have seen that ions are formed when atoms gain or lose electrons. Also, hund's rule still applies. Electrons are typically. Electron Removal Order.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Removal Order Ions are formed when atoms gain or lose electrons. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. Also, hund's rule still applies. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. This means that electrons will. Electron Removal Order.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Electron Removal Order Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. Also, hund's rule still applies. The electrons will be removed from the highest. Electron Removal Order.
From courses.lumenlearning.com
Electrons Biology for Majors I Electron Removal Order The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; For main group elements, the electrons that were added last are the first electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are.. Electron Removal Order.
From instrumentationtools.com
Difference between Electron and Proton Electron Removal Order Ions are formed when atoms gain or lose electrons. For transition metals and inner transition metals, however, electrons in the s orbital are. We have seen that ions are formed when atoms gain or lose electrons. This means that electrons will be removed from the 4s subshell before any electrons are. The electron removed during the ionization of beryllium ([he]2s. Electron Removal Order.
From pressbooks.bccampus.ca
1.5 Electronic Structure of Atoms (Electron Configurations) Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron. Electron Removal Order.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Electron Removal Order Ions are formed when atoms gain or lose electrons. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; We have seen that ions are formed when atoms gain or lose electrons. The electrons will be removed from the. Electron Removal Order.
From www.readchemistry.com
Molecular Orbitals for Homonuclear Diatomic Molecules (MO Theory Electron Removal Order This means that electrons will be removed from the 4s subshell before any electrons are. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. For transition metals and inner transition metals, however, electrons in the s orbital are. A cation (positively charged ion) forms. Electron Removal Order.
From www.alamy.com
Ionization energy (IE) Amount of energy required to remove the most Electron Removal Order The electrons will be removed from the highest energy shell in the neutral configuration. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Electrons are typically removed from the valence shells, which are the highest s and p. Electron Removal Order.
From socratic.org
When electrons ocoupy different orbitals of the same sublevel, do they Electron Removal Order The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; The electrons will be removed from the highest energy shell in the neutral configuration. This means that electrons will be removed from the 4s subshell before any electrons are.. Electron Removal Order.
From www.pinterest.com
78 best images about Chemistry Electron Configuration on Pinterest Electron Removal Order For transition metals and inner transition metals, however, electrons in the s orbital are. A cation (positively charged ion) forms when one or more electrons are removed from a. We have seen that ions are formed when atoms gain or lose electrons. Electrons are typically removed from the valence shells, which are the highest s and p orbitals. These are. Electron Removal Order.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Electron Removal Order A cation (positively charged ion) forms when one or more electrons are removed from a. A cation (positively charged ion) forms when one or more electrons are removed. These are the electrons in the highest energy level, and so it is logical that they will be removed first when the scandium forms ions. The electron removed during the ionization of. Electron Removal Order.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Electron Removal Order We have seen that ions are formed when atoms gain or lose electrons. The electrons will be removed from the highest energy shell in the neutral configuration. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Ions are. Electron Removal Order.