Cl Ion Atomic Radius . The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si).
from www.doubtnut.com
Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration.
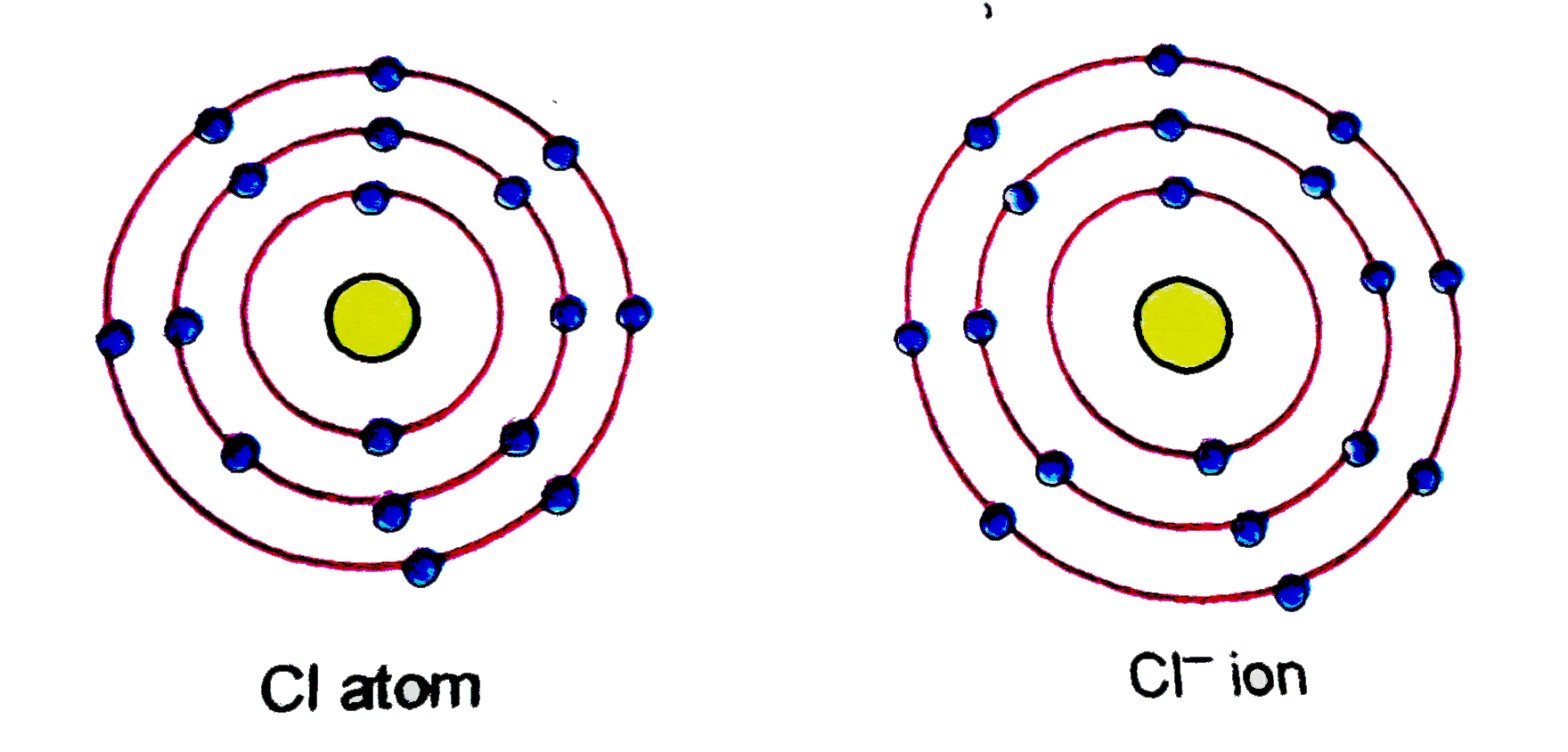
(a) Give the schematic atomic structures of chlorine atom and chloride
Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration.
From knordslearning.com
Atomic Radius Trend in Periodic Table (Simple Explanation) Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From pediaa.com
Difference Between Atomic Radius and Ionic Radius Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From www.breakingatom.com
Atomic Radius of Elements Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From pediaa.com
Difference Between Atomic Radius and Ionic Radius Definition Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From exoykphwz.blob.core.windows.net
Chlorine Electrons Number at Linwood Bell blog Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID4493576 Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From 2012books.lardbucket.org
Sizes of Atoms and Ions Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From www.chemistrylearner.com
Ionic Radius Definition, Examples, Chart, & Periodic Trend Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Cl Ion Atomic Radius.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Cl Ion Atomic Radius.
From exyurxnox.blob.core.windows.net
Sodium Chlorine Atomic Radius at Charles Dunn blog Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From anthonystrendsassignment.weebly.com
Ionic Radius Periodic Trends Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From ar.inspiredpencil.com
Periodic Table Ionic Radius Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From pubchem.ncbi.nlm.nih.gov
Atomic Radius Periodic Table of Elements PubChem Cl Ion Atomic Radius The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From www.periodictableprintable.com
Interactive Periodic Table Showing Atomic Radius 2024 Periodic Table Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Cl Ion Atomic Radius The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From pediabay.com
Atomic Radius of Elements (With Periodic table Chart) Pediabay Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Cl Ion Atomic Radius.
From www.slideserve.com
PPT Chemistry periodicity Atomic and Ionic Radius PowerPoint Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Cl Ion Atomic Radius.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From 88guru.com
Atomic RadiusAn Overview 88Guru Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From sciencenotes.org
Chlorine Facts Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From animalia-life.club
Atomic Radius Diagram Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From www.animalia-life.club
Ionic Radius Diagram Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Cl Ion Atomic Radius.
From general.chemistrysteps.com
Ionic Radius Chemistry Steps Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From www.crystalmaker.com
Elements, Atomic Radii and the Periodic Radii Cl Ion Atomic Radius The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From retiky.weebly.com
retiky Blog Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From ar.inspiredpencil.com
Periodic Table Ionic Radius Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Shannon, revised effective ionic radii and systematic studies of interatomic. The data contained in the database was taken from: Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Cl Ion Atomic Radius.
From www.pinterest.com
What is the difference between the ionic radius and the atomic radius Cl Ion Atomic Radius The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Cl Ion Atomic Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Cl Ion Atomic Radius Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Shannon, revised effective ionic radii and systematic studies of interatomic. Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Cl Ion Atomic Radius.
From www.chem.fsu.edu
Electron Configurations Cl Ion Atomic Radius Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). The data contained in the database was taken from: Shannon, revised effective ionic radii and systematic studies of interatomic. Cl Ion Atomic Radius.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Cl Ion Atomic Radius Shannon, revised effective ionic radii and systematic studies of interatomic. Carbon and silicon are both in group 14 with carbon lying above, so carbon is smaller than silicon (c < si). Ionic radius varies with the charge of the ion (and number of electrons) and the electron configuration. The data contained in the database was taken from: Cl Ion Atomic Radius.