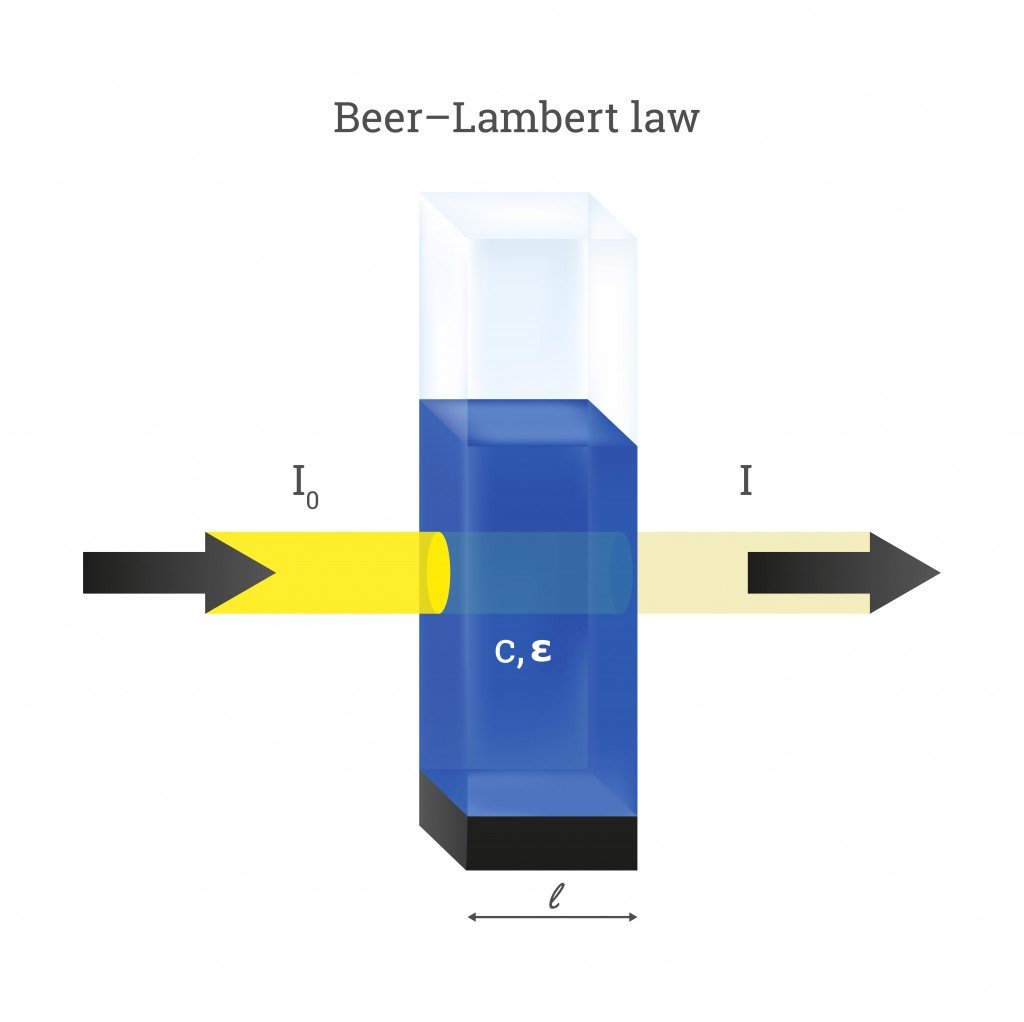
Beer's Law In Simple Terms . beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.
from www.scienceabc.com
Beer’s law is a limiting law that is valid only for low concentrations of analyte. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. fundamental limitations to beer's law.
Beers Law Definition, History, Equation, Formula And Example
Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte.
From exolwzeip.blob.core.windows.net
Beer's Law Equation Chemistry at Taylor Jones blog Beer's Law In Simple Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional. Beer's Law In Simple Terms.
From www.researchgate.net
Beer's law verification range for method A (a) and Method B (b Beer's Law In Simple Terms beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. fundamental limitations to beer's law. beer’s law, in spectroscopy, a relation concerning the absorption. Beer's Law In Simple Terms.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law In Simple Terms beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. . Beer's Law In Simple Terms.
From www.scribd.com
Application of Beer’s Law Quantitative analysis for a single analyte Beer's Law In Simple Terms The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law In Simple Terms.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law In Simple Terms There are two contributions to this. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation. Beer's Law In Simple Terms.
From www.youtube.com
Deviations from BeerLambert law YouTube Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy. Beer's Law In Simple Terms.
From www.studocu.com
IMA Beers LAW complete beer s law Lambert’s law when a Beer's Law In Simple Terms fundamental limitations to beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. The amount of energy absorbed or transmitted by a solution. Beer's Law In Simple Terms.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law In Simple Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is directly proportional. Beer's Law In Simple Terms.
From www.youtube.com
Beer's Law and it's Mathematical Expressions YouTube Beer's Law In Simple Terms There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. fundamental limitations to beer's law. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. beer’s law, in spectroscopy, a relation. Beer's Law In Simple Terms.
From exorencaa.blob.core.windows.net
Beer's Law Breakdown at Paul Virgil blog Beer's Law In Simple Terms fundamental limitations to beer's law. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law states that the light absorbance of a. Beer's Law In Simple Terms.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law In Simple Terms fundamental limitations to beer's law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. Beer’s law is a limiting law that is valid only. Beer's Law In Simple Terms.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law In Simple Terms beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium.. Beer's Law In Simple Terms.
From www.researchgate.net
Beer's law curves for method A and method B Download Scientific Diagram Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law In Simple Terms.
From www.studocu.com
Beer's law prelab Pre laboratory assignment for Beer's Law Beer's Law In Simple Terms The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the. Beer's Law In Simple Terms.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law In Simple Terms There are two contributions to this. Beer’s law is a limiting law that is valid only for low concentrations of analyte. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law In Simple Terms.
From www.reddit.com
Visualization of Beer’s law in beer r/chemistry Beer's Law In Simple Terms The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. There are two contributions to this. fundamental limitations to beer's law. Beer’s law is a limiting law that. Beer's Law In Simple Terms.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. fundamental limitations to beer's law. Beer’s law is a limiting law that. Beer's Law In Simple Terms.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Beer's Law In Simple Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. . Beer's Law In Simple Terms.
From robot.ekstrabladet.dk
Lei De Lambert Beer Beer's Law In Simple Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. There are two contributions to this. fundamental limitations to beer's law. The amount of energy absorbed or transmitted by. Beer's Law In Simple Terms.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law In Simple Terms beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly. Beer's Law In Simple Terms.
From www.slideserve.com
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer Beer's Law In Simple Terms beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. fundamental limitations to beer's law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer’s law is a limiting law that. Beer's Law In Simple Terms.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law In Simple Terms fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. in spectroscopy, beer’s law states that the absorption of light by a. Beer's Law In Simple Terms.
From www.chegg.com
Solved Describe what each term in the Beer's Law equation Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. Beer’s law is a limiting law that is valid only for low concentrations of analyte. . Beer's Law In Simple Terms.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law In Simple Terms There are two contributions to this. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. . Beer's Law In Simple Terms.
From www.youtube.com
beer law sample problems YouTube Beer's Law In Simple Terms fundamental limitations to beer's law. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The amount of energy absorbed or transmitted by a solution is. Beer's Law In Simple Terms.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species. Beer's Law In Simple Terms.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law In Simple Terms beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. There are two contributions to this. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of. Beer's Law In Simple Terms.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The amount of energy absorbed or transmitted by a solution is proportional to the solution's molar absorptivity and the. Beer’s law is a limiting law that is valid only for low concentrations of analyte. beer’s law, in. Beer's Law In Simple Terms.
From www.studocu.com
Lab 4 Beer's Law Lab 4 Beer’s Law OBJECTIVES. The purpose of this Beer's Law In Simple Terms in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is. Beer's Law In Simple Terms.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. There are two contributions to this. Beer’s law is a limiting law that is valid only. Beer's Law In Simple Terms.
From www.studypool.com
SOLUTION Beer lambert law, definition, history, derivation, diagram Beer's Law In Simple Terms There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.. Beer's Law In Simple Terms.
From www.studocu.com
Beers Law Lab Ph ET Student Sheet AP Chemistry PhET Simulation Beer Beer's Law In Simple Terms since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There. Beer's Law In Simple Terms.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law In Simple Terms in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. There are two contributions to this. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low. Beer's Law In Simple Terms.
From www.youtube.com
Lambert Beer Law YouTube Beer's Law In Simple Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. fundamental limitations to beer's law. There are two contributions to this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of. Beer's Law In Simple Terms.
From www.chegg.com
Solved Part 1 Beer's Law and Mass Absorption Coefficients Beer's Law In Simple Terms in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. fundamental limitations to beer's law. beer’s law states that the light absorbance of a solution is. Beer's Law In Simple Terms.