Between Magnesium And Calcium Which One Is More Reactive . Magnesium, aluminium and zinc can react with water, but. 58 rows there is some ambiguity at the borderlines between the groups. The alkali metals, barium, radium, strontium, and calcium react with cold water. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Students will be able to discover that group 1 metals are more reactive than group 2. 9 rows the reactivity series ranks metals by how readily they react. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. More reactive metals displace less reactive metals from their compounds and. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. Magnesium is more active than aluminum. Magnesium only slowly reacts with cold water, but. Noble metals are the least reactive.
from www.slideserve.com
Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. 58 rows there is some ambiguity at the borderlines between the groups. Noble metals are the least reactive. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Magnesium, aluminium and zinc can react with water, but. Magnesium is more active than aluminum. Magnesium only slowly reacts with cold water, but. More reactive metals displace less reactive metals from their compounds and. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time.
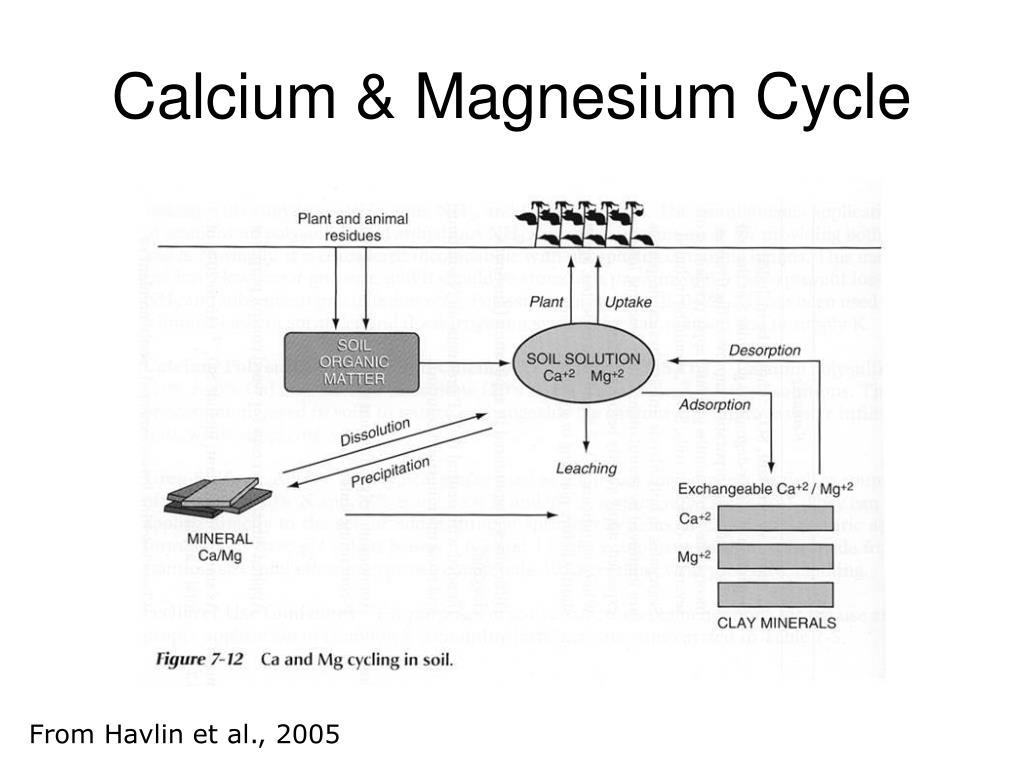
PPT Ca, Mg and S in Soil PowerPoint Presentation, free download ID
Between Magnesium And Calcium Which One Is More Reactive Students will be able to discover that group 1 metals are more reactive than group 2. Magnesium, aluminium and zinc can react with water, but. 9 rows the reactivity series ranks metals by how readily they react. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Students will be able to discover that group 1 metals are more reactive than group 2. 58 rows there is some ambiguity at the borderlines between the groups. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. More reactive metals displace less reactive metals from their compounds and. The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium only slowly reacts with cold water, but. Noble metals are the least reactive. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Magnesium is more active than aluminum.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Between Magnesium And Calcium Which One Is More Reactive Noble metals are the least reactive. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium. Between Magnesium And Calcium Which One Is More Reactive.
From www.tldrpharmacy.com
Hyperkalemia An Overview for Pharmacists — tl;dr pharmacy Between Magnesium And Calcium Which One Is More Reactive Magnesium only slowly reacts with cold water, but. 9 rows the reactivity series ranks metals by how readily they react. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Noble metals are the least reactive.. Between Magnesium And Calcium Which One Is More Reactive.
From agutsygirl.com
Types of Magnesium {Your Master Guide} A Gutsy Girl® Between Magnesium And Calcium Which One Is More Reactive Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Students will be able to discover that group 1 metals are more reactive than group 2. The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium is more active than aluminum. Noble metals are the least reactive. More. Between Magnesium And Calcium Which One Is More Reactive.
From www.slideserve.com
PPT Ca, Mg and S in Soil PowerPoint Presentation, free download ID Between Magnesium And Calcium Which One Is More Reactive 58 rows there is some ambiguity at the borderlines between the groups. Magnesium, aluminium and zinc can react with water, but. More reactive metals displace less reactive metals from their compounds and. Magnesium only slowly reacts with cold water, but. Magnesium is more active than aluminum. Students will be able to discover that group 1 metals are more reactive than. Between Magnesium And Calcium Which One Is More Reactive.
From supplementsalon.com
Calcium Magnesium Ratio Unlocking The Secret Of 21 To Stronger Bones Between Magnesium And Calcium Which One Is More Reactive When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Magnesium, aluminium and zinc can react with water, but. More reactive metals displace less reactive metals from their compounds and. Magnesium only slowly reacts with cold water, but. Magnesium is more active than aluminum. Compare group 1 and group 2 metals with. Between Magnesium And Calcium Which One Is More Reactive.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Between Magnesium And Calcium Which One Is More Reactive Magnesium is more active than aluminum. More reactive metals displace less reactive metals from their compounds and. 9 rows the reactivity series ranks metals by how readily they react. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. When reacting magnesium with hcl, it started to. Between Magnesium And Calcium Which One Is More Reactive.
From thepaleodiet.com
The Importance of the Magnesium and Calcium Relationship A Key Ratio Between Magnesium And Calcium Which One Is More Reactive Magnesium only slowly reacts with cold water, but. Magnesium is more active than aluminum. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. 58 rows there is some ambiguity at the borderlines between the groups. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Calcium. Between Magnesium And Calcium Which One Is More Reactive.
From material-properties.org
Magnesium and Calcium Comparison Properties Material Properties Between Magnesium And Calcium Which One Is More Reactive The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium is more active than aluminum. Noble metals are the least reactive. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. 9 rows the reactivity series ranks metals by how readily they react. Magnesium, aluminium and zinc can. Between Magnesium And Calcium Which One Is More Reactive.
From www.semanticscholar.org
Figure 2 from Regulation of calcium and magnesium homeostasis in plants Between Magnesium And Calcium Which One Is More Reactive 9 rows the reactivity series ranks metals by how readily they react. 58 rows there is some ambiguity at the borderlines between the groups. Magnesium only slowly reacts with cold water, but. Magnesium, aluminium and zinc can react with water, but. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Calcium. Between Magnesium And Calcium Which One Is More Reactive.
From www.differencebetween.com
Difference Between Calcium and Magnesium Compare the Difference Between Magnesium And Calcium Which One Is More Reactive 58 rows there is some ambiguity at the borderlines between the groups. The alkali metals, barium, radium, strontium, and calcium react with cold water. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. Magnesium is more active than aluminum. Calcium is more reactive than magnesium because. Between Magnesium And Calcium Which One Is More Reactive.
From www.dreamstime.com
Magnesium and Calcium in Balance Pictured As a Scale and Words Between Magnesium And Calcium Which One Is More Reactive Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium, aluminium and zinc can react with water, but. Magnesium is more active than aluminum. More reactive metals displace less reactive metals from their compounds and. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Calcium. Between Magnesium And Calcium Which One Is More Reactive.
From www.differencebetween.com
Difference Between Calcium and Magnesium Compare the Difference Between Magnesium And Calcium Which One Is More Reactive Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Magnesium is more active than aluminum. 9 rows the reactivity series ranks metals by how readily they react. Students will be able to discover that group 1 metals are more reactive than group 2. When reacting magnesium with hcl, it started. Between Magnesium And Calcium Which One Is More Reactive.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Between Magnesium And Calcium Which One Is More Reactive Magnesium only slowly reacts with cold water, but. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. 9 rows the reactivity series ranks metals by how readily they react. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. The alkali metals, barium, radium, strontium,. Between Magnesium And Calcium Which One Is More Reactive.
From www.mdpi.com
Nutrients Free FullText Magnesium in Prevention and Therapy Between Magnesium And Calcium Which One Is More Reactive When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium, aluminium and zinc can react with water, but. 9 rows the reactivity series ranks metals by how readily they react. 58 rows there is some ambiguity. Between Magnesium And Calcium Which One Is More Reactive.
From www.semanticscholar.org
Physiology of Calcium, Phosphate, Magnesium and Vitamin D. Semantic Between Magnesium And Calcium Which One Is More Reactive Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Noble metals are the least reactive. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. 9 rows the reactivity series ranks metals by how readily they react. Students will be able to. Between Magnesium And Calcium Which One Is More Reactive.
From www.pinterest.com
Your Guide To All Of The Different Magnesium Supplements Out There Between Magnesium And Calcium Which One Is More Reactive When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Students will be able to discover that group 1 metals are more reactive than group 2. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. More reactive metals. Between Magnesium And Calcium Which One Is More Reactive.
From www.researchgate.net
Calcium and Magnesium Cycle. Download Scientific Diagram Between Magnesium And Calcium Which One Is More Reactive The alkali metals, barium, radium, strontium, and calcium react with cold water. Noble metals are the least reactive. Magnesium only slowly reacts with cold water, but. 58 rows there is some ambiguity at the borderlines between the groups. More reactive metals displace less reactive metals from their compounds and. Calcium and the metals that are more reactive than calcium in. Between Magnesium And Calcium Which One Is More Reactive.
From www.grassrootshealth.net
Understanding the Balance between Magnesium, Calcium and Vitamin D Between Magnesium And Calcium Which One Is More Reactive Students will be able to discover that group 1 metals are more reactive than group 2. The alkali metals, barium, radium, strontium, and calcium react with cold water. More reactive metals displace less reactive metals from their compounds and. Magnesium, aluminium and zinc can react with water, but. 58 rows there is some ambiguity at the borderlines between the groups.. Between Magnesium And Calcium Which One Is More Reactive.
From www.grassrootshealth.net
Understanding the Balance between Magnesium, Calcium and Vitamin D Between Magnesium And Calcium Which One Is More Reactive Magnesium only slowly reacts with cold water, but. 9 rows the reactivity series ranks metals by how readily they react. The alkali metals, barium, radium, strontium, and calcium react with cold water. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Calcium and the metals that are more reactive than. Between Magnesium And Calcium Which One Is More Reactive.
From www.youtube.com
CHEMISTRY 9/CHAPTER 8/LECTURE 8/CHEMICAL REACTIVITY/ USES OF METALS Between Magnesium And Calcium Which One Is More Reactive Compare group 1 and group 2 metals with this practical that shows their reactivity rates. More reactive metals displace less reactive metals from their compounds and. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Magnesium only slowly reacts with cold water, but. Magnesium is more active than aluminum. Students will. Between Magnesium And Calcium Which One Is More Reactive.
From www.researchgate.net
Doseresponse relationship between blood calcium, magnesium Between Magnesium And Calcium Which One Is More Reactive Magnesium, aluminium and zinc can react with water, but. More reactive metals displace less reactive metals from their compounds and. Students will be able to discover that group 1 metals are more reactive than group 2. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. 58 rows there is some ambiguity. Between Magnesium And Calcium Which One Is More Reactive.
From www.elektramagnesium.com.au
Protect Against Cardiovascular Disease With Magnesium Elektra Magnesium Between Magnesium And Calcium Which One Is More Reactive Magnesium only slowly reacts with cold water, but. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. 9 rows the reactivity series ranks metals by how readily they react. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Between Magnesium And Calcium Which One Is More Reactive.
From thepaleodiet.com
3 Micronutrient Ratios to Keep in Balance The Paleo Diet® Between Magnesium And Calcium Which One Is More Reactive 58 rows there is some ambiguity at the borderlines between the groups. Noble metals are the least reactive. Magnesium, aluminium and zinc can react with water, but. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. The alkali metals, barium, radium, strontium, and calcium react with cold water. When reacting. Between Magnesium And Calcium Which One Is More Reactive.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Between Magnesium And Calcium Which One Is More Reactive More reactive metals displace less reactive metals from their compounds and. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. Magnesium only slowly reacts with cold water, but. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it. Between Magnesium And Calcium Which One Is More Reactive.
From www.ncbi.nlm.nih.gov
Figure 4, Relationships between magnesium, calcium, and sodium content Between Magnesium And Calcium Which One Is More Reactive 9 rows the reactivity series ranks metals by how readily they react. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Noble metals are the least reactive. Magnesium is more active than aluminum. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold. Between Magnesium And Calcium Which One Is More Reactive.
From quizdbcornwallis.z21.web.core.windows.net
What Makes An Element More Reactive Between Magnesium And Calcium Which One Is More Reactive Students will be able to discover that group 1 metals are more reactive than group 2. Magnesium, aluminium and zinc can react with water, but. Noble metals are the least reactive. The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium only slowly reacts with cold water, but. More reactive metals displace less reactive metals from their. Between Magnesium And Calcium Which One Is More Reactive.
From clinicalnutritionespen.com
Relationships between serum and dietary magnesium, calcium, and Between Magnesium And Calcium Which One Is More Reactive Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Magnesium is more active than aluminum. More reactive metals displace less reactive metals from their compounds and. Students will be able to discover that group 1 metals are more reactive than group 2. Calcium and the metals that are more reactive. Between Magnesium And Calcium Which One Is More Reactive.
From www.reddit.com
Which type of Magnesium is best? How large of a dose is Between Magnesium And Calcium Which One Is More Reactive When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. The alkali metals, barium, radium, strontium, and calcium react with cold water. More reactive metals displace less reactive metals from their compounds and. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. 9 rows the reactivity. Between Magnesium And Calcium Which One Is More Reactive.
From www.pinterest.com
The Magnesium Calcium Relationship Calcium, Protein synthesis, Magnesium Between Magnesium And Calcium Which One Is More Reactive Noble metals are the least reactive. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. 9 rows the reactivity series ranks metals by how readily they react. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. When reacting magnesium with. Between Magnesium And Calcium Which One Is More Reactive.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Between Magnesium And Calcium Which One Is More Reactive 9 rows the reactivity series ranks metals by how readily they react. When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Students will be able to discover that group 1 metals are more reactive than group. Between Magnesium And Calcium Which One Is More Reactive.
From www.youtube.com
Magnesium and Calcium (Part 3) Hypercalcemia Causes & How To Get Rid Between Magnesium And Calcium Which One Is More Reactive 58 rows there is some ambiguity at the borderlines between the groups. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. Magnesium, aluminium and zinc can react. Between Magnesium And Calcium Which One Is More Reactive.
From stock.adobe.com
Magnesium and calcium in balance pictured as words Magnesium, calcium Between Magnesium And Calcium Which One Is More Reactive Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Compare group 1 and group 2 metals with this practical that shows their reactivity rates. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. 58 rows there. Between Magnesium And Calcium Which One Is More Reactive.
From www.agproud.com
Calcium and magnesium are important to the success of the transition Between Magnesium And Calcium Which One Is More Reactive Noble metals are the least reactive. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. The alkali metals, barium, radium, strontium, and calcium react with cold water. Magnesium is more active than aluminum. Magnesium only slowly reacts with cold water, but. Compare group 1 and group 2 metals with this. Between Magnesium And Calcium Which One Is More Reactive.
From utedzz.blogspot.com
Periodic Table Magnesium Element Symbol Periodic Table Timeline Between Magnesium And Calcium Which One Is More Reactive When reacting magnesium with hcl, it started to slowly fiz after we let it react for some time. 9 rows the reactivity series ranks metals by how readily they react. Magnesium is more active than aluminum. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. The. Between Magnesium And Calcium Which One Is More Reactive.
From www.pinterest.com
Figure 166.1 from 166 Calcium, Magnesium, and Phosphorus Semantic Between Magnesium And Calcium Which One Is More Reactive Noble metals are the least reactive. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding. Magnesium, aluminium and zinc can react with water, but. Calcium is more reactive than magnesium because calcium has a lower electron affinity and ionization energy, making it easier. Magnesium only slowly. Between Magnesium And Calcium Which One Is More Reactive.