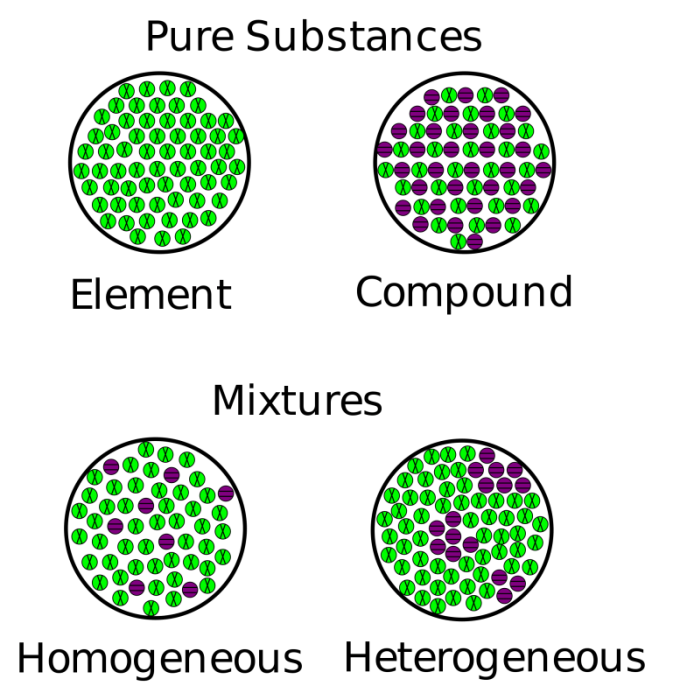
Compound Mixture Or Element . Explain the difference between a homogeneous mixture and a. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. In a compound, elements are chemically bonded. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Explain the difference between an element and a compound. They do not contain any atomic bonds. A compound is a pure substance that is made from more than one element. Matter is anything that has mass and volume. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. For example, we mentioned that oxygen is a compound as it.
from manualworshipped.z14.web.core.windows.net
Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Matter is anything that has mass and volume. In a compound, elements are chemically bonded. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Explain the difference between a homogeneous mixture and a. They do not contain any atomic bonds. A compound is a pure substance that is made from more than one element. For example, we mentioned that oxygen is a compound as it. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound.
Mixture Of Two Compounds Diagram
Compound Mixture Or Element In a compound, elements are chemically bonded. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). For example, we mentioned that oxygen is a compound as it. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. They do not contain any atomic bonds. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Explain the difference between a homogeneous mixture and a. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. In a compound, elements are chemically bonded. Matter is anything that has mass and volume. Explain the difference between an element and a compound. A compound is a pure substance that is made from more than one element.
From slidetodoc.com
Pure Substances and Mixtures Categorizing Matter All matter Compound Mixture Or Element In a compound, elements are chemically bonded. They do not contain any atomic bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. A compound is a pure substance that is made from more than one element. Explain the difference between a homogeneous mixture and a.. Compound Mixture Or Element.
From www.tes.com
Elements, compounds and mixtures Teaching Resources Compound Mixture Or Element Matter is anything that has mass and volume. For example, we mentioned that oxygen is a compound as it. In a compound, elements are chemically bonded. Explain the difference between a homogeneous mixture and a. Explain the difference between an element and a compound. Looking at the left side of the chart, we can see that a pure substance can. Compound Mixture Or Element.
From www.slideserve.com
PPT Elements, Compounds and Mixtures PowerPoint Presentation, free Compound Mixture Or Element Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Matter is anything that has mass and volume. They do not contain any atomic bonds. Explain the difference between a homogeneous. Compound Mixture Or Element.
From manualworshipped.z14.web.core.windows.net
Mixture Of Two Compounds Diagram Compound Mixture Or Element A compound is a pure substance that is made from more than one element. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Explain the difference between a homogeneous mixture and a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Compound Mixture Or Element.
From sites.google.com
Unit 4 Elements, Compounds, and Mixtures Ms. Evangelista's MS Science Compound Mixture Or Element Explain the difference between an element and a compound. They do not contain any atomic bonds. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound is a pure substance that is made from more than one element. Mixtures are made of two or more substances — elements or compounds — that. Compound Mixture Or Element.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet Compound Mixture Or Element Explain the difference between an element and a compound. For example, we mentioned that oxygen is a compound as it. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Matter is anything that has mass and volume. In a compound, elements are chemically bonded. Looking at the left side of the chart, we. Compound Mixture Or Element.
From schematicfixpulpits.z21.web.core.windows.net
Diagram Of Mixtures Elements And Compounds Compound Mixture Or Element For example, we mentioned that oxygen is a compound as it. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Matter is anything that has mass and volume. They do not contain any atomic bonds. In a compound, elements are chemically bonded. Microscopic view of a gaseous mixture containing. Compound Mixture Or Element.
From www.youtube.com
Elements Compounds Mixtures YouTube Compound Mixture Or Element A compound is a pure substance that is made from more than one element. They do not contain any atomic bonds. In a compound, elements are chemically bonded. Matter is anything that has mass and volume. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Looking at the left. Compound Mixture Or Element.
From mungfali.com
Element Compound And Mixture Chart Compound Mixture Or Element In a compound, elements are chemically bonded. Explain the difference between an element and a compound. They do not contain any atomic bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. For example, we mentioned that oxygen is a compound as it. Explain the difference. Compound Mixture Or Element.
From studylib.net
Element, compound or mixture Compound Mixture Or Element Explain the difference between a homogeneous mixture and a. They do not contain any atomic bonds. Matter is anything that has mass and volume. Explain the difference between an element and a compound. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Classify a substance as. Compound Mixture Or Element.
From teachingthekid.blogspot.com
Teaching the Kid Elements, Compounds, and Mixtures Compound Mixture Or Element A compound is a pure substance that is made from more than one element. In a compound, elements are chemically bonded. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Explain the difference between a homogeneous mixture and a. Looking at the left side of the chart, we can see that a pure substance. Compound Mixture Or Element.
From www.shutterstock.com
Different Between Elements Compounds Mixtures Example Stock Compound Mixture Or Element They do not contain any atomic bonds. In a compound, elements are chemically bonded. Explain the difference between a homogeneous mixture and a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Matter is anything that has mass. Compound Mixture Or Element.
From learningmagicpurlicues.z13.web.core.windows.net
Element Compound And Mixture Compound Mixture Or Element Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). They do not contain any atomic bonds. A compound is a pure substance that is made from more than one element. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound.. Compound Mixture Or Element.
From www.slideserve.com
PPT Elements, Compounds, Mixtures PowerPoint Presentation, free Compound Mixture Or Element Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). They do not contain any atomic bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Mixtures are made of two or more substances — elements or compounds — that. Compound Mixture Or Element.
From www.slideserve.com
PPT Elements, Molecules, Compounds, and Mixtures PowerPoint Compound Mixture Or Element In a compound, elements are chemically bonded. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Explain the difference between a homogeneous mixture and a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Looking at the left side of the chart, we can see that a. Compound Mixture Or Element.
From manualworshipped.z14.web.core.windows.net
Element Compound Mixture Diagram Compound Mixture Or Element In a compound, elements are chemically bonded. Explain the difference between an element and a compound. They do not contain any atomic bonds. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound is a pure substance that is made from more than one element. Mixtures are made of two or more. Compound Mixture Or Element.
From www.numerade.com
SOLVED 10 POINTS!!!! Classify each as either element compound mixture Compound Mixture Or Element Matter is anything that has mass and volume. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Explain the difference between a homogeneous mixture and a. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; In a compound, elements are chemically bonded. Explain. Compound Mixture Or Element.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Compound Mixture Or Element Matter is anything that has mass and volume. They do not contain any atomic bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Explain the difference between a homogeneous mixture and a. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and. Compound Mixture Or Element.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element Compound Mixture Or Element Matter is anything that has mass and volume. Explain the difference between a homogeneous mixture and a. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound is a pure substance that is made from more than one element. Mixtures are made of two or more substances — elements or compounds —. Compound Mixture Or Element.
From mavink.com
Element Compound And Mixture Examples Compound Mixture Or Element Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Explain the difference between an element and a compound. In a compound, elements are chemically bonded. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Looking at the left side of the chart,. Compound Mixture Or Element.
From herbalic79.blogspot.com
Element Compound Mixture Diagram Worksheet Herbalic Compound Mixture Or Element Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). For example, we mentioned that oxygen is a compound as it. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. In a compound, elements are chemically bonded. They do not contain any atomic bonds. Explain the difference between. Compound Mixture Or Element.
From stock.adobe.com
Stockillustratie classification of matter mixture (homogeneous and Compound Mixture Or Element Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Matter is anything that has mass and volume. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; In a compound, elements are chemically bonded. A compound is a pure substance that is made from. Compound Mixture Or Element.
From guidemanualephemerist.z14.web.core.windows.net
Element Compound Mixture Diagram Compound Mixture Or Element For example, we mentioned that oxygen is a compound as it. They do not contain any atomic bonds. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; In a compound, elements are chemically bonded.. Compound Mixture Or Element.
From www.tes.com
Element, Compound, Mixture Bundle Teaching Resources Compound Mixture Or Element Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. They do not contain any atomic bonds. A compound is a pure substance that is made from more than one element. In a compound, elements are chemically bonded. Looking at the left side of the chart, we can see that a pure substance can be. Compound Mixture Or Element.
From studylib.net
Elements, Compounds, and Mixtures Compound Mixture Or Element They do not contain any atomic bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Matter is anything that has mass and volume. A compound is a pure substance that is made from more than one element. Classify a substance as an element, a compound,. Compound Mixture Or Element.
From studylib.net
element compound mixture matching pairs Compound Mixture Or Element Matter is anything that has mass and volume. Explain the difference between a homogeneous mixture and a. Explain the difference between an element and a compound. In a compound, elements are chemically bonded. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). For example, we mentioned that oxygen is a compound as it.. Compound Mixture Or Element.
From www.vrogue.co
The Difference Between Elements Compounds And Mixture vrogue.co Compound Mixture Or Element Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). A compound is a pure substance that is made from more than one element. They do not contain any atomic bonds. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. In a compound, elements are chemically bonded. Matter. Compound Mixture Or Element.
From www.majordifferences.com
6 Differences between Compounds and Mixtures with examples Compound Mixture Or Element Matter is anything that has mass and volume. A compound is a pure substance that is made from more than one element. They do not contain any atomic bonds. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. For example, we mentioned that oxygen is a compound as it. Explain the difference between an. Compound Mixture Or Element.
From www.slideshare.net
Elements, Compounds, Mixtures Compound Mixture Or Element Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. In a compound, elements are chemically bonded. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Matter is anything that has mass and volume. Explain the difference between an element and. Compound Mixture Or Element.
From www.youtube.com
Elements, Compounds, Mixtures YouTube Compound Mixture Or Element They do not contain any atomic bonds. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Explain the difference between a homogeneous mixture and a. In a compound, elements are chemically bonded. Looking at the left side of the chart, we can see that a pure substance can be. Compound Mixture Or Element.
From www.youtube.com
Pure Substances, Elements, Compounds, Homogenous & Heterogenous Mixture Compound Mixture Or Element Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; Explain the difference between an element and a compound. Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). Looking at the left side of the chart, we can see that a pure substance. Compound Mixture Or Element.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID2199820 Compound Mixture Or Element Matter is anything that has mass and volume. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Mixtures are made of two or more substances — elements or compounds — that are mixed physically but not chemically; For example, we mentioned that oxygen is a compound as it. Explain the difference between an element. Compound Mixture Or Element.
From quizlet.com
Shell TOPIC 2 Elements, compounds and mixtures Diagram Quizlet Compound Mixture Or Element A compound is a pure substance that is made from more than one element. For example, we mentioned that oxygen is a compound as it. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Explain the difference between an element and a compound. Matter is anything that has mass and volume. Explain the difference. Compound Mixture Or Element.
From circuitdiagramalexandra.z5.web.core.windows.net
Element Compound Mixture Diagram Compound Mixture Or Element Matter is anything that has mass and volume. Explain the difference between an element and a compound. A compound is a pure substance that is made from more than one element. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. For example, we mentioned that oxygen is a compound as it. In a compound,. Compound Mixture Or Element.
From www.dreamstime.com
Elements, Mixtures, and Compounds Compared Stock Vector Illustration Compound Mixture Or Element Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. For example, we mentioned that oxygen is a compound as it. Microscopic view of a gaseous mixture containing two elements (argon and. Compound Mixture Or Element.