Calorimetry Lab Discussion . Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. As part of this lab, you will: For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Lab session 9, experiment 8: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Sl syllabus, written by the chemistry experts at save my exams. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic.
from www.studocu.com
Sl syllabus, written by the chemistry experts at save my exams. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Lab session 9, experiment 8: Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. As part of this lab, you will: To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of.
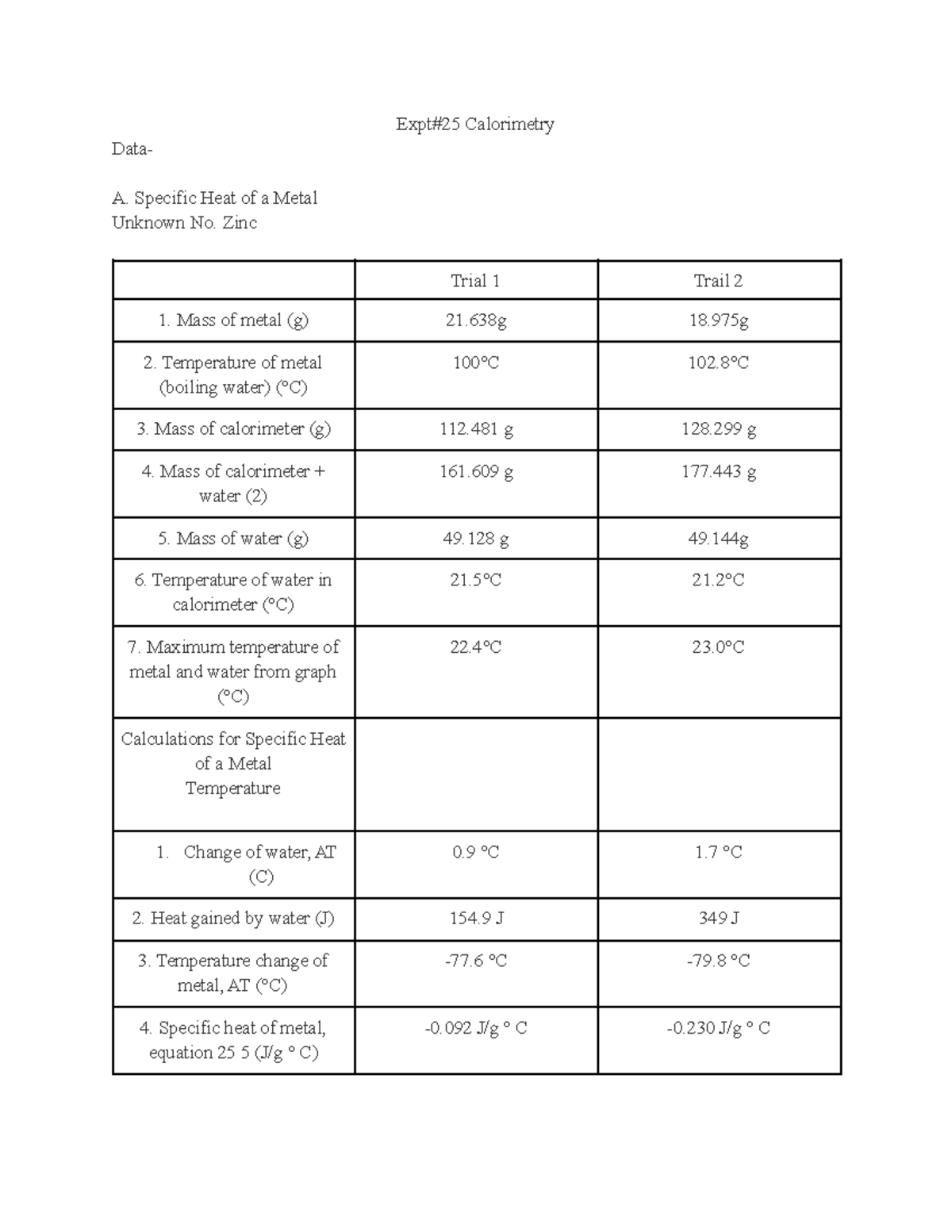
Expt25 Calorimetry lab report Expt25 Calorimetry Data A
Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Sl syllabus, written by the chemistry experts at save my exams. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. As part of this lab, you will: To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic.
From pdfprof.com
experiment 25 calorimetry lab report Calorimetry Lab Discussion Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and. Calorimetry Lab Discussion.
From www.youtube.com
L3 Calorimetry sheet discussion by manish sir YouTube Calorimetry Lab Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from. Calorimetry Lab Discussion.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Sl syllabus, written by the chemistry experts at save my exams. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. As part of this lab, you will:. Calorimetry Lab Discussion.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Calorimetry Lab Discussion Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Sl syllabus, written by the chemistry experts at save. Calorimetry Lab Discussion.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Discussion Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Lab session 9, experiment 8: Sl syllabus, written by the chemistry experts at save my exams. As part of this lab,. Calorimetry Lab Discussion.
From www.youtube.com
CHEM103 Lab 5 Calorimetry [PreLab Discussion] 2Mar2023 YouTube Calorimetry Lab Discussion Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. As part of this lab, you will: Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. In this experiment you will heat a known mass of a metal to a known. Calorimetry Lab Discussion.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Lab session 9, experiment 8: Abstract the main purpose of the calorimetry experiment is. Calorimetry Lab Discussion.
From www.scribd.com
Calorimetry Lab Explanation Chemistry Physical Sciences Free 30 Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. To. Calorimetry Lab Discussion.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Lab Discussion Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Sl syllabus, written by the chemistry experts at save my exams. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. In this experiment you will. Calorimetry Lab Discussion.
From cityraven.com
🎉 Heat effects and calorimetry lab. Calorimeter. 20190203 Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of.. Calorimetry Lab Discussion.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Sl syllabus, written by the chemistry experts at save my exams.. Calorimetry Lab Discussion.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Discussion Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Calorimetry is based on. Calorimetry Lab Discussion.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Lab Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Lab session 9, experiment 8: As part of this lab, you will: For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry is based on the first law of thermodynamics that states that energy cannot. Calorimetry Lab Discussion.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Discussion Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. As part of this lab,. Calorimetry Lab Discussion.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. Sl syllabus, written by the chemistry experts at save my exams. Calorimetry is based on the first law of. Calorimetry Lab Discussion.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. In this experiment you will heat a known mass of a metal. Calorimetry Lab Discussion.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. As part of this lab, you will: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor. Calorimetry Lab Discussion.
From www.studocu.com
Lab 6 Calorimetry Wu. Name Partner Student No Student No Lab Calorimetry Lab Discussion Sl syllabus, written by the chemistry experts at save my exams. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. As part of this lab, you will: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Calorimetry is based on the first law of thermodynamics. Calorimetry Lab Discussion.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Calorimetry Lab Discussion As part of this lab, you will: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry Lab Discussion.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorimetry Lab Discussion Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Lab session 9, experiment 8: For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. As part of this lab,. Calorimetry Lab Discussion.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup Calorimetry Lab Discussion Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction.. Calorimetry Lab Discussion.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Discussion Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Sl syllabus, written. Calorimetry Lab Discussion.
From www.youtube.com
Soda Can Calorimeter Lab YouTube Calorimetry Lab Discussion Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Sl syllabus, written by the chemistry experts at save my exams. As part of this lab, you will: Revision notes on 5.1.4 calorimetry experiments for the dp. Calorimetry Lab Discussion.
From browsegrades.net
Get Great Study Materials and Great Services Calorimetry Lab Discussion Lab session 9, experiment 8: Sl syllabus, written by the chemistry experts at save my exams. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic.. Calorimetry Lab Discussion.
From www.studocu.com
Calorimetry Lab Calorimetry Lab (1 week) (LCA) Calorimetry Purpose Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. In. Calorimetry Lab Discussion.
From www.studocu.com
Copy of Optional Gizmo Calorimetry Student Exploration Calorimetry Calorimetry Lab Discussion Sl syllabus, written by the chemistry experts at save my exams. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Lab session 9,. Calorimetry Lab Discussion.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorimetry Lab Discussion Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. As part of this lab, you will: Calorimetry is based on. Calorimetry Lab Discussion.
From studylib.net
Calorimetry and Coffee Cups Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed.. Calorimetry Lab Discussion.
From www.studocu.com
Expt25 Calorimetry lab report Expt25 Calorimetry Data A Calorimetry Lab Discussion To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Lab session 9, experiment 8: As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Calorimetry is based on. Calorimetry Lab Discussion.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Discussion For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Lab session 9, experiment 8: To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. Revision notes on 5.1.4 calorimetry experiments for the dp ib chemistry: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity,. Calorimetry Lab Discussion.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Lab Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. As part of this lab, you will: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that. To remind you, endothermic reactions gain energy from the surroundings (δh is. Calorimetry Lab Discussion.
From users.highland.edu
Calorimetry Calorimetry Lab Discussion Ccal represents the heat capacity of the calorimeter and it is measured to calculate the heat of any other reaction taking place in that specific calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. In this experiment you will heat a known mass of a metal to. Calorimetry Lab Discussion.
From www.studocu.com
7.03H Calorimetry lab Module 7 Honors Calorimetry Activity Use the Calorimetry Lab Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. As part of this lab, you will: Lab session 9, experiment 8: To remind you, endothermic reactions gain energy from the surroundings (δh is positive) and exothermic. In this experiment you will heat a known mass of a metal. Calorimetry Lab Discussion.
From courses.lumenlearning.com
Calorimetry General Chemistry Calorimetry Lab Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. Calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. As part of this lab, you will: For chemical reactions, we can use. Calorimetry Lab Discussion.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Discussion As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known amount of. For chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Calorimetry, heat of reaction specific heat is an intensive property of. Calorimetry Lab Discussion.