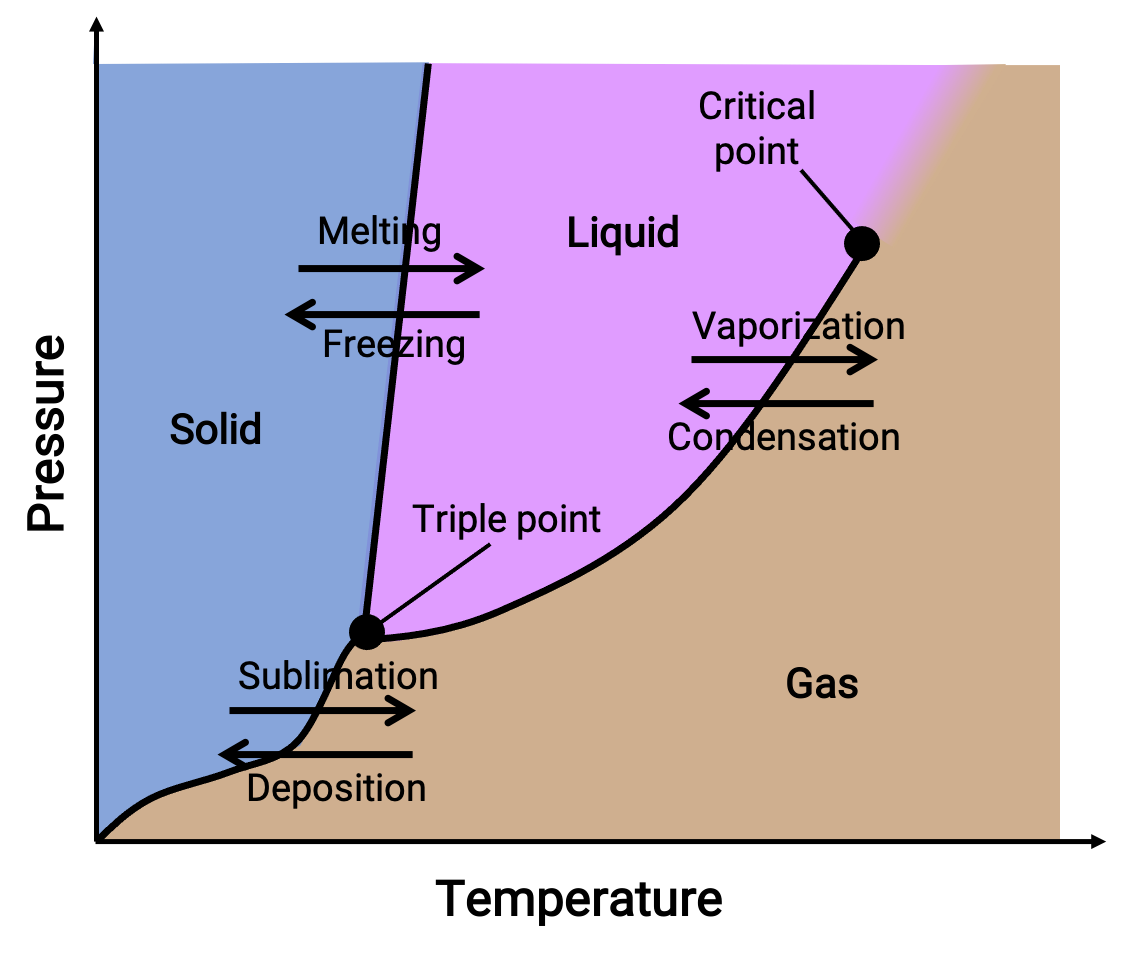
Physical Chemistry Solid-Liquid Equilibrium . A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. • the interface between phases: Understand the types of physical equilibrium i.e. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. These are solids, liquids, and gases. The areas of physical equilibria we will investigate are: There are three particular phases between which we will be examining discrete phase changes. The solid and liquid regions are separated by the. Solution properties like freezing and boiling. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Physical equilibrium develops between different phases or physical properties.
from unistudium.unipg.it
The areas of physical equilibria we will investigate are: These are solids, liquids, and gases. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Understand the types of physical equilibrium i.e. The solid and liquid regions are separated by the. Physical equilibrium develops between different phases or physical properties. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Solution properties like freezing and boiling. • the interface between phases: Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases.
Phase Diagrams
Physical Chemistry Solid-Liquid Equilibrium Solution properties like freezing and boiling. The areas of physical equilibria we will investigate are: Solution properties like freezing and boiling. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Physical equilibrium develops between different phases or physical properties. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. There are three particular phases between which we will be examining discrete phase changes. The solid and liquid regions are separated by the. These are solids, liquids, and gases. Understand the types of physical equilibrium i.e. • the interface between phases:
From www.pinterest.com
Chemistry education, Physics and mathematics, Chemistry Physical Chemistry Solid-Liquid Equilibrium Physical equilibrium develops between different phases or physical properties. There are three particular phases between which we will be examining discrete phase changes. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid,. Physical Chemistry Solid-Liquid Equilibrium.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: The areas of physical equilibria we will investigate are: Understand the types of physical equilibrium i.e. These are solids, liquids, and gases. Solution properties like freezing and boiling. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Physical equilibrium develops between different phases or physical properties. Explain the types of physical. Physical Chemistry Solid-Liquid Equilibrium.
From learncheme.com
ssleequilibrium LearnChemE Physical Chemistry Solid-Liquid Equilibrium A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. The areas of physical equilibria we will investigate are: These are solids, liquids, and gases. • the interface between phases: Solution properties like freezing and boiling. Understand the types of physical equilibrium i.e. Explain the types of. Physical Chemistry Solid-Liquid Equilibrium.
From slideplayer.com
Chapter 17 Equilibrium Reversible Reactions. ppt download Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: Solution properties like freezing and boiling. Physical equilibrium develops between different phases or physical properties. The areas of physical equilibria we will investigate are: These are solids, liquids, and gases. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Explain the. Physical Chemistry Solid-Liquid Equilibrium.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: There are three particular phases between which we will be examining discrete phase changes. These are solids, liquids, and gases. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and. Physical Chemistry Solid-Liquid Equilibrium.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Physical Chemistry Solid-Liquid Equilibrium Solution properties like freezing and boiling. These are solids, liquids, and gases. Physical equilibrium develops between different phases or physical properties. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. The solid and liquid regions are separated by the. There are three particular phases. Physical Chemistry Solid-Liquid Equilibrium.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Physical Chemistry Solid-Liquid Equilibrium Understand the types of physical equilibrium i.e. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. The solid and liquid regions are separated by the. The areas of physical equilibria we will investigate are: Physical equilibrium develops between different phases or physical properties. These are solids,. Physical Chemistry Solid-Liquid Equilibrium.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium Physical Chemistry Solid-Liquid Equilibrium These are solids, liquids, and gases. There are three particular phases between which we will be examining discrete phase changes. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. • the interface between phases: The solid and liquid regions are separated by the. Physical equilibrium develops. Physical Chemistry Solid-Liquid Equilibrium.
From www.toppr.com
Equilibrium in Physical Processes SolidLiquidGas Equilibrium, Examples Physical Chemistry Solid-Liquid Equilibrium Physical equilibrium develops between different phases or physical properties. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Understand the types of physical equilibrium i.e. The areas of physical equilibria we will investigate are: These are solids, liquids, and gases. The solid and liquid. Physical Chemistry Solid-Liquid Equilibrium.
From joijcatto.blob.core.windows.net
Changing From Liquid To Solid at Leo Smiley blog Physical Chemistry Solid-Liquid Equilibrium Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. These are solids, liquids, and gases. Physical equilibrium develops between different phases or physical properties. Solution properties like freezing and boiling. The areas of physical equilibria we will investigate are: Phase diagrams contain discrete regions. Physical Chemistry Solid-Liquid Equilibrium.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: The solid and liquid regions are separated by the. These are solids, liquids, and gases. There are three particular phases between which we will be examining discrete phase changes. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Solution properties like. Physical Chemistry Solid-Liquid Equilibrium.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: The areas of physical equilibria we will investigate are: Solution properties like freezing and boiling. The solid and liquid regions are separated by the. Physical equilibrium develops between different phases or physical properties. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at. Physical Chemistry Solid-Liquid Equilibrium.
From www.snexplores.org
Explainer What are the different states of matter? Physical Chemistry Solid-Liquid Equilibrium The areas of physical equilibria we will investigate are: Physical equilibrium develops between different phases or physical properties. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. These are solids, liquids, and gases. Understand the types of physical equilibrium i.e. • the interface between. Physical Chemistry Solid-Liquid Equilibrium.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Physical Chemistry Solid-Liquid Equilibrium These are solids, liquids, and gases. • the interface between phases: Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. There are three particular phases between which we will be examining discrete phase changes. Solution properties like freezing and boiling. A key tool in exploring phase equilibria is a phase diagram which is. Physical Chemistry Solid-Liquid Equilibrium.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Physical Chemistry Solid-Liquid Equilibrium The solid and liquid regions are separated by the. There are three particular phases between which we will be examining discrete phase changes. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. A key tool in exploring phase equilibria is a phase diagram which. Physical Chemistry Solid-Liquid Equilibrium.
From guidediagramordures.z1.web.core.windows.net
How To Draw A Phase Diagram Physical Chemistry Solid-Liquid Equilibrium There are three particular phases between which we will be examining discrete phase changes. Understand the types of physical equilibrium i.e. Physical equilibrium develops between different phases or physical properties. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The areas of physical equilibria we will investigate are: • the interface between phases: A key tool. Physical Chemistry Solid-Liquid Equilibrium.
From shaunmwilliams.com
Chapter 1 Presentation Physical Chemistry Solid-Liquid Equilibrium A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The solid and liquid regions are separated by the. These are solids, liquids, and gases. Explain the types of physical equilibrium, equilibrium of solids. Physical Chemistry Solid-Liquid Equilibrium.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Physical Chemistry Solid-Liquid Equilibrium These are solids, liquids, and gases. The solid and liquid regions are separated by the. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Physical equilibrium develops between different phases or physical properties. The areas of physical equilibria we will investigate are: Solution properties like freezing and boiling. Understand the types of physical equilibrium i.e. There. Physical Chemistry Solid-Liquid Equilibrium.
From studylib.net
Equilibrium for a general reaction Physical Chemistry Solid-Liquid Equilibrium Understand the types of physical equilibrium i.e. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. There are three particular phases between which we will be examining discrete phase changes. Physical equilibrium develops between different phases or physical properties. Phase diagrams contain discrete regions. Physical Chemistry Solid-Liquid Equilibrium.
From www.mindomo.com
Fluids review Mind Map Physical Chemistry Solid-Liquid Equilibrium Understand the types of physical equilibrium i.e. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. • the interface between phases: The areas of physical equilibria we will investigate are: The solid and liquid regions are separated by the. Solution properties like freezing and boiling. Explain. Physical Chemistry Solid-Liquid Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Physical Chemistry Solid-Liquid Equilibrium Solution properties like freezing and boiling. There are three particular phases between which we will be examining discrete phase changes. These are solids, liquids, and gases. The solid and liquid regions are separated by the. • the interface between phases: Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in. Physical Chemistry Solid-Liquid Equilibrium.
From ecampusontario.pressbooks.pub
Chemical Equilibrium First Year General Chemistry Physical Chemistry Solid-Liquid Equilibrium Understand the types of physical equilibrium i.e. The solid and liquid regions are separated by the. • the interface between phases: Solution properties like freezing and boiling. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Physical equilibrium develops between different phases or physical. Physical Chemistry Solid-Liquid Equilibrium.
From www.slideserve.com
PPT Chem 1310 Introduction to physical chemistry Part 3 Equilibria Physical Chemistry Solid-Liquid Equilibrium The solid and liquid regions are separated by the. Physical equilibrium develops between different phases or physical properties. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. • the interface between phases: A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Explain. Physical Chemistry Solid-Liquid Equilibrium.
From mungfali.com
Solid Solution Phase Diagram Physical Chemistry Solid-Liquid Equilibrium A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. There are three particular phases between which we will be examining discrete phase changes. • the interface between phases: Solution properties like freezing and boiling. These are solids, liquids, and gases. Physical equilibrium develops between different phases. Physical Chemistry Solid-Liquid Equilibrium.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Physical Chemistry Solid-Liquid Equilibrium There are three particular phases between which we will be examining discrete phase changes. Understand the types of physical equilibrium i.e. The solid and liquid regions are separated by the. The areas of physical equilibria we will investigate are: Physical equilibrium develops between different phases or physical properties. Solution properties like freezing and boiling. These are solids, liquids, and gases.. Physical Chemistry Solid-Liquid Equilibrium.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the Physical Chemistry Solid-Liquid Equilibrium Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. • the interface between phases: A key tool in exploring phase equilibria is a phase diagram which is used to show conditions. Physical Chemistry Solid-Liquid Equilibrium.
From unistudium.unipg.it
Phase Diagrams Physical Chemistry Solid-Liquid Equilibrium There are three particular phases between which we will be examining discrete phase changes. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Physical equilibrium develops between different phases or physical properties. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. •. Physical Chemistry Solid-Liquid Equilibrium.
From gradeup.co
NEET 2020 Study Notes Equilibrium Physical Chemistry Solid-Liquid Equilibrium Understand the types of physical equilibrium i.e. The solid and liquid regions are separated by the. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Physical equilibrium develops between different phases or physical properties. These are solids, liquids, and gases. • the interface between phases: The. Physical Chemistry Solid-Liquid Equilibrium.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Physical Chemistry Solid-Liquid Equilibrium Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. These are solids, liquids, and gases. Solution properties like freezing and boiling. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The areas of physical equilibria we will investigate are: A. Physical Chemistry Solid-Liquid Equilibrium.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.1.1 The Three States of Matter翰林国际教育 Physical Chemistry Solid-Liquid Equilibrium Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. • the interface between phases: The solid and liquid regions are separated by the. Understand the types of physical equilibrium i.e. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. These. Physical Chemistry Solid-Liquid Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Physical Chemistry Solid-Liquid Equilibrium The solid and liquid regions are separated by the. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The areas of physical equilibria we will investigate are: Understand the types of. Physical Chemistry Solid-Liquid Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Physical Chemistry Solid-Liquid Equilibrium Solution properties like freezing and boiling. The solid and liquid regions are separated by the. Physical equilibrium develops between different phases or physical properties. Understand the types of physical equilibrium i.e. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and. Physical Chemistry Solid-Liquid Equilibrium.
From www.flexiprep.com
Chemistry Class 11 NCERT Solutions Chapter 7 Equilibrium Part 2 Physical Chemistry Solid-Liquid Equilibrium • the interface between phases: The areas of physical equilibria we will investigate are: Physical equilibrium develops between different phases or physical properties. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. Explain. Physical Chemistry Solid-Liquid Equilibrium.
From www.peoi.org
Chapter 1 Section A Some Basic Definitions Physical Chemistry Solid-Liquid Equilibrium Explain the types of physical equilibrium, equilibrium of solids and liquids, vapour and liquid, solids and gases, solids dissolving in liquid and gaseous dissolution in. A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. Understand the types of physical equilibrium i.e. • the interface between phases:. Physical Chemistry Solid-Liquid Equilibrium.
From achs-prod.acs.org
SolidLiquid Phase Equilibrium and Thermodynamic Analysis of N,N Physical Chemistry Solid-Liquid Equilibrium A key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume, etc.) at which. These are solids, liquids, and gases. • the interface between phases: Understand the types of physical equilibrium i.e. Phase diagrams contain discrete regions corresponding to the solid, liquid, and gas phases. The areas of physical equilibria we. Physical Chemistry Solid-Liquid Equilibrium.