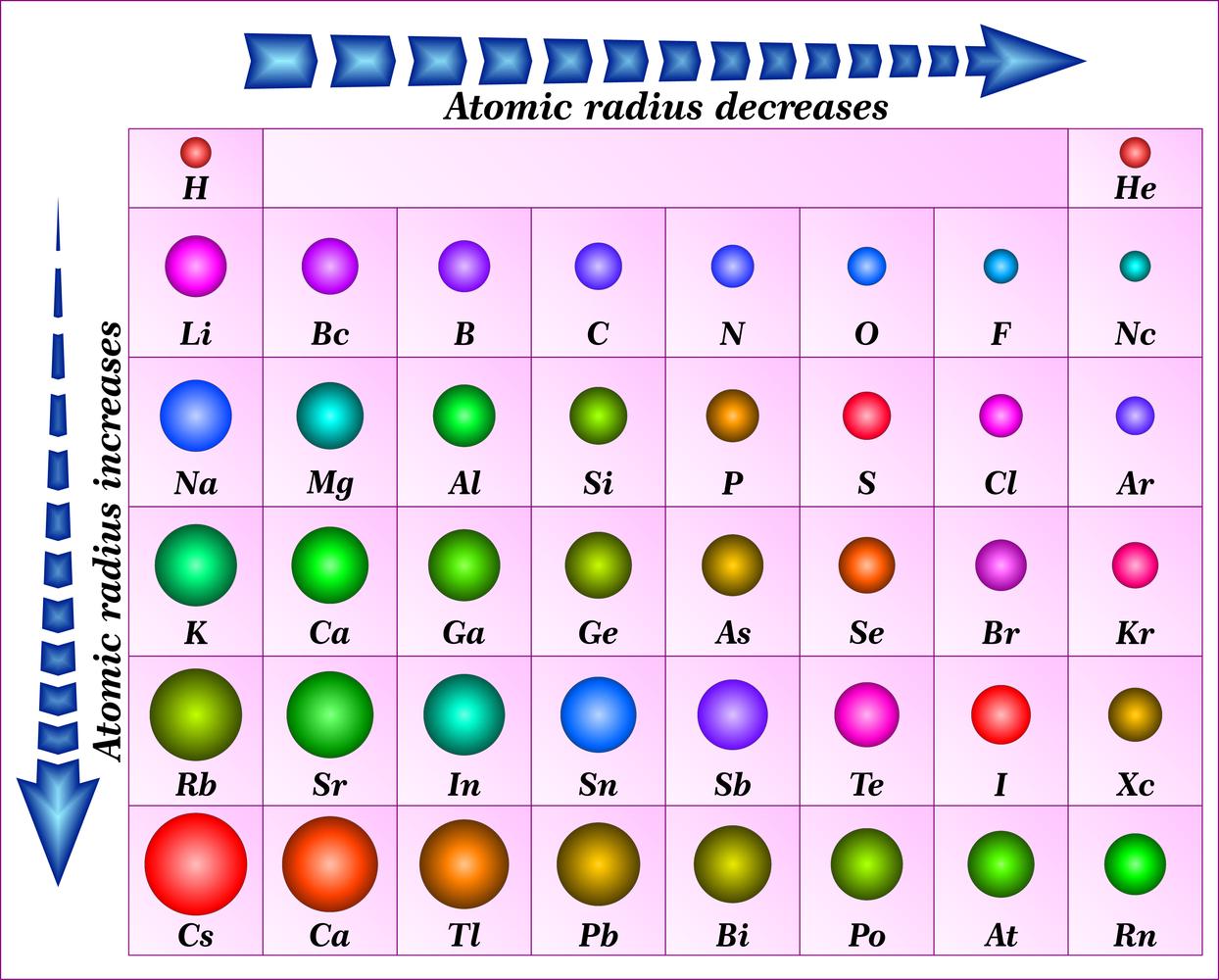
What Is The Measuring Unit Of Atomic Radius . 1 å = 1 × 10−10 m = 100 pm. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Because an atom does not have a. Atomic radii can be measured by. In simpler terms, it can be defined as something similar to the radius of a circle,. We define the atomic radius of a chemical element as: Definitions of the atomic radius. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. (a) the covalent atomic radius, rcov, is half the distance.
from 88guru.com
The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. (a) the covalent atomic radius, rcov, is half the distance. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Because an atom does not have a. We define the atomic radius of a chemical element as: Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Definitions of the atomic radius. In simpler terms, it can be defined as something similar to the radius of a circle,. 1 å = 1 × 10−10 m = 100 pm. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron.
Atomic RadiusAn Overview 88Guru
What Is The Measuring Unit Of Atomic Radius 1 å = 1 × 10−10 m = 100 pm. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Because an atom does not have a. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. We define the atomic radius of a chemical element as: Definitions of the atomic radius. In simpler terms, it can be defined as something similar to the radius of a circle,. Atomic radii can be measured by. 1 å = 1 × 10−10 m = 100 pm. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. (a) the covalent atomic radius, rcov, is half the distance. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius Because an atom does not have a. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. (a) the covalent atomic radius, rcov, is half the distance. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron.. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius 1 å = 1 × 10−10 m = 100 pm. In simpler terms, it can be defined as something similar to the radius of a circle,. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The type of atomic radius being measured here is called the metallic. What Is The Measuring Unit Of Atomic Radius.
From www.trentu.ca
PreChemistry What Is The Measuring Unit Of Atomic Radius Because an atom does not have a. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Atomic radii can be measured by. Definitions of the atomic. What Is The Measuring Unit Of Atomic Radius.
From www.pngwing.com
Atomradius Kovalenter Radius Periodentrends Chemie, Atomgrößentrend What Is The Measuring Unit Of Atomic Radius Definitions of the atomic radius. We define the atomic radius of a chemical element as: The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. 1 å = 1 × 10−10 m = 100 pm. The type of atomic radius. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius (a) the covalent atomic radius, rcov, is half the distance. In simpler terms, it can be defined as something similar to the radius of a circle,. Because an atom does not have a. Definitions of the atomic radius. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron.. What Is The Measuring Unit Of Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) What Is The Measuring Unit Of Atomic Radius 1 å = 1 × 10−10 m = 100 pm. We define the atomic radius of a chemical element as: Atomic radii can be measured by. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The atomic radius of a chemical element is a measure of the size of. What Is The Measuring Unit Of Atomic Radius.
From www.youtube.com
Atomic Radius Example 2 YouTube What Is The Measuring Unit Of Atomic Radius (a) the covalent atomic radius, rcov, is half the distance. In simpler terms, it can be defined as something similar to the radius of a circle,. Definitions of the atomic radius. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The atomic radius of a chemical element. What Is The Measuring Unit Of Atomic Radius.
From www.shutterstock.com
Illustration Chemistry Atomic Radius Measure Size Stock Vector (Royalty What Is The Measuring Unit Of Atomic Radius The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. In simpler terms, it can be defined as something similar to the radius of a circle,. 1 å = 1 × 10−10 m = 100 pm. (a) the covalent atomic. What Is The Measuring Unit Of Atomic Radius.
From pediabay.com
Atomic Radius of Elements (With Periodic table Chart) Pediabay What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. 1 å = 1 × 10−10 m = 100 pm. Atomic radii can be. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius (a) the covalent atomic radius, rcov, is half the distance. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. In simpler terms, it can be defined as. What Is The Measuring Unit Of Atomic Radius.
From www.slideserve.com
PPT Unit 2 20142015 PowerPoint Presentation, free download ID What Is The Measuring Unit Of Atomic Radius The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Definitions of the atomic radius. 1 å = 1 × 10−10 m = 100 pm. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the. What Is The Measuring Unit Of Atomic Radius.
From www.youtube.com
Finding radius and volume of atom from density and molar mass YouTube What Is The Measuring Unit Of Atomic Radius Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. In simpler terms, it can be defined as something similar to the radius of a circle,. Definitions of the atomic radius. The type of atomic radius being measured here is called the metallic radius or the covalent radius. What Is The Measuring Unit Of Atomic Radius.
From proper-cooking.info
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Atomic radii can be measured by. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Definitions of. What Is The Measuring Unit Of Atomic Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. Because an atom does not have a. We define the atomic radius of a chemical element as: 1 å = 1 × 10−10 m = 100 pm. (a) the covalent atomic radius, rcov, is half the distance. The type of atomic radius being measured. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius We define the atomic radius of a chemical element as: (a) the covalent atomic radius, rcov, is half the distance. In simpler terms, it can be defined as something similar to the radius of a circle,. Definitions of the atomic radius. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of. What Is The Measuring Unit Of Atomic Radius.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General What Is The Measuring Unit Of Atomic Radius Definitions of the atomic radius. 1 å = 1 × 10−10 m = 100 pm. Because an atom does not have a. We define the atomic radius of a chemical element as: In simpler terms, it can be defined as something similar to the radius of a circle,. Atomic radius is generally stated as being the total distance from an. What Is The Measuring Unit Of Atomic Radius.
From www.sliderbase.com
Atoms, molecules and ions Presentation Chemistry What Is The Measuring Unit Of Atomic Radius Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost. What Is The Measuring Unit Of Atomic Radius.
From in.pinterest.com
Atomic radius Ionic radius, Chemistry education, Chemistry What Is The Measuring Unit Of Atomic Radius The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. 1 å = 1 × 10−10 m = 100 pm. We define the atomic radius of a chemical element as: Atomic radii can be measured by. (a) the covalent atomic radius, rcov, is half the distance. Atomic radius or. What Is The Measuring Unit Of Atomic Radius.
From 88guru.com
Atomic RadiusAn Overview 88Guru What Is The Measuring Unit Of Atomic Radius Definitions of the atomic radius. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. We define the atomic radius of a chemical element as: Because an atom does. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius We define the atomic radius of a chemical element as: The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Because an atom does not have a. The type of atomic radius being measured here is called the metallic radius. What Is The Measuring Unit Of Atomic Radius.
From www.flinnsci.ca
Atomic Sizes and Radii Chart Flinn Scientific What Is The Measuring Unit Of Atomic Radius The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Definitions of the atomic radius. Atomic radii can be measured by. Because an atom does not have a. (a) the covalent atomic radius, rcov, is half the distance. In simpler. What Is The Measuring Unit Of Atomic Radius.
From www.pearson.com
Given the atomic radius of the elementsDetermine the atomic radiu What Is The Measuring Unit Of Atomic Radius Atomic radii can be measured by. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The atomic radius of a chemical element is a measure of the. What Is The Measuring Unit Of Atomic Radius.
From www.breakingatom.com
Atomic Radius of Elements What Is The Measuring Unit Of Atomic Radius Because an atom does not have a. 1 å = 1 × 10−10 m = 100 pm. Atomic radii can be measured by. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. We define the atomic radius of a chemical element as: Atomic radius or atomic radii is the. What Is The Measuring Unit Of Atomic Radius.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy What Is The Measuring Unit Of Atomic Radius 1 å = 1 × 10−10 m = 100 pm. Definitions of the atomic radius. (a) the covalent atomic radius, rcov, is half the distance. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Because an atom does not have a. Atomic radius is generally stated as. What Is The Measuring Unit Of Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) What Is The Measuring Unit Of Atomic Radius Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. 1 å = 1 × 10−10 m = 100 pm. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron.. What Is The Measuring Unit Of Atomic Radius.
From pubchem.ncbi.nlm.nih.gov
Atomic Radius Periodic Table of Elements PubChem What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. Atomic radii can be measured by. Because an atom does not have a. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. (a) the covalent atomic radius, rcov, is half the. What Is The Measuring Unit Of Atomic Radius.
From www.shutterstock.com
How Measure Atomic Radius Distance Between vector de stock (libre de What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. (a) the covalent atomic radius, rcov, is half the distance. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. 1 å = 1. What Is The Measuring Unit Of Atomic Radius.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 What Is The Measuring Unit Of Atomic Radius Atomic radii can be measured by. 1 å = 1 × 10−10 m = 100 pm. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. (a). What Is The Measuring Unit Of Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) What Is The Measuring Unit Of Atomic Radius We define the atomic radius of a chemical element as: In simpler terms, it can be defined as something similar to the radius of a circle,. The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. Because an atom does not have a. 1 å = 1 × 10−10. What Is The Measuring Unit Of Atomic Radius.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps What Is The Measuring Unit Of Atomic Radius We define the atomic radius of a chemical element as: Definitions of the atomic radius. Atomic radii can be measured by. In simpler terms, it can be defined as something similar to the radius of a circle,. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of. What Is The Measuring Unit Of Atomic Radius.
From surfguppy.com
What is Electronegativity? What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. Definitions of the atomic radius. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Atomic radii can be measured by. Atomic radius or. What Is The Measuring Unit Of Atomic Radius.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is The Measuring Unit Of Atomic Radius The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. We define the atomic radius of a chemical element as: Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of. What Is The Measuring Unit Of Atomic Radius.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID What Is The Measuring Unit Of Atomic Radius Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. In simpler terms, it can be defined as something similar to the radius of a circle,. We define. What Is The Measuring Unit Of Atomic Radius.
From 2012books.lardbucket.org
Sizes of Atoms and Ions What Is The Measuring Unit Of Atomic Radius (a) the covalent atomic radius, rcov, is half the distance. Definitions of the atomic radius. Atomic radii can be measured by. We define the atomic radius of a chemical element as: The type of atomic radius being measured here is called the metallic radius or the covalent radius depending on the bonding. In simpler terms, it can be defined as. What Is The Measuring Unit Of Atomic Radius.
From triviakids.blogspot.com
Atomic Radius Of Lead Trivia for Kids What Is The Measuring Unit Of Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle,. Because an atom does not have a. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is generally stated as being the total distance from an atom’s nucleus to. What Is The Measuring Unit Of Atomic Radius.