Titration Introduction Chemistry Khan Academy . In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're behind a web filter, please. This unit is part of the chemistry library. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).
from www.youtube.com
If you're seeing this message, it means we're having trouble loading external resources on our website. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). This unit is part of the chemistry library. If you're behind a web filter, please. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).
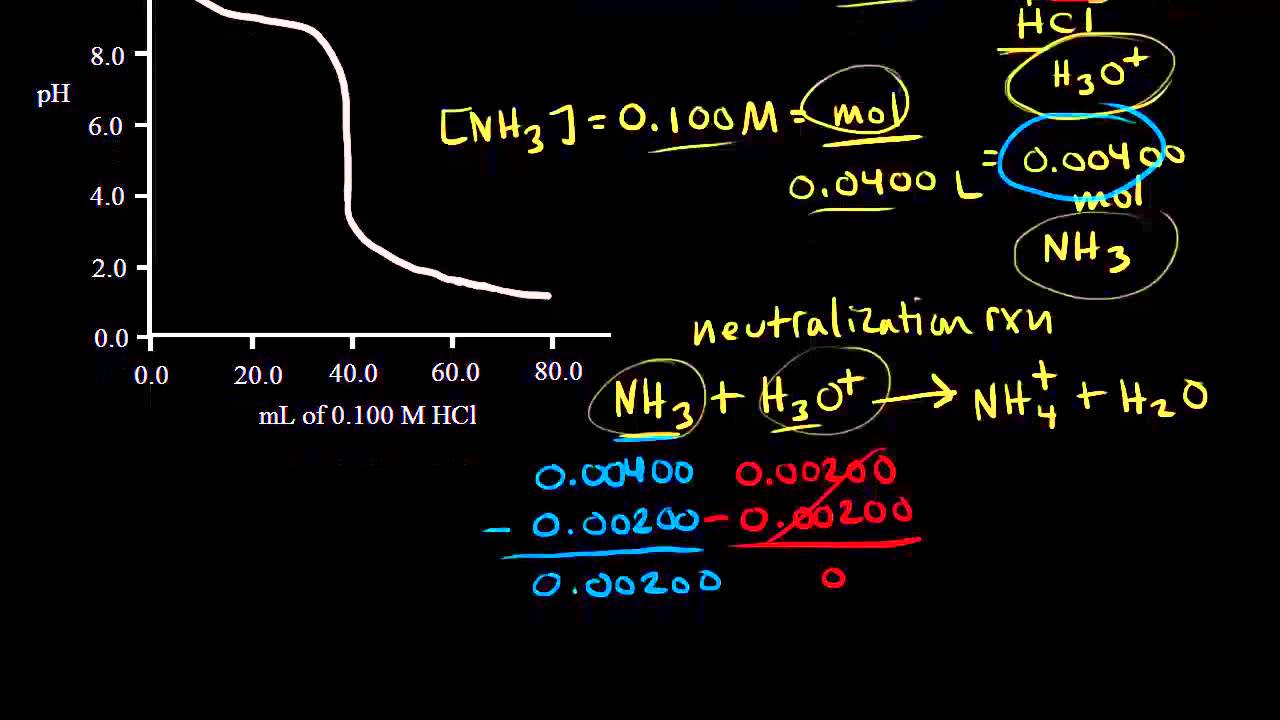
Titration of a weak base with a strong acid Chemistry Khan Academy
Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. This unit is part of the chemistry library. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. If you're behind a web filter, please. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website.
From hxectggyv.blob.core.windows.net
Back Titration Khan Academy at Elizabeth Walker blog Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please.. Titration Introduction Chemistry Khan Academy.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Introduction Chemistry Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. This unit is part of the. Titration Introduction Chemistry Khan Academy.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. If you're seeing this message, it means we're having trouble loading external resources on our website. This unit is part of the chemistry library. If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).. Titration Introduction Chemistry Khan Academy.
From www.studypool.com
SOLUTION Acid base titration curves titrations chemical processes mcat Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're behind a web filter, please. This unit is part of the chemistry library.. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration roundup Buffers, titrations, and solubility equilibria Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. This unit. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Filtration Is matter around us pure? Chemistry Khan Academy YouTube Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration of a weak base with a strong acid Chemistry Khan Academy Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). This unit is part of the chemistry library. If you're seeing this message, it means we're. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration introduction Chemistry Khan Academy YouTube Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. If you're seeing this message, it means we're having. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration curves and acidbase indicators Chemistry Khan Academy Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. This unit is part of the chemistry library. If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website.. Titration Introduction Chemistry Khan Academy.
From www.studypool.com
SOLUTION Acid base titration curves titrations chemical processes mcat Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're behind a web filter, please. Browse videos, articles, and exercises by topic. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified. Titration Introduction Chemistry Khan Academy.
From hxectggyv.blob.core.windows.net
Back Titration Khan Academy at Elizabeth Walker blog Titration Introduction Chemistry Khan Academy In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're behind a web filter, please. This unit is part of the chemistry library. In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Weak acidstrong base titrations Acids and bases AP Chemistry Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're behind a web filter, please. In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.lessonplanet.com
Khan Academy Acid Base Titration Example Instructional Video for 9th Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). This unit is part of the chemistry library. In a titration, a solution of known concentration (the titrant) is added to a solution of. Titration Introduction Chemistry Khan Academy.
From www.khanacademy.org
Acidbase titrations (practice) Khan Academy Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). This unit. Titration Introduction Chemistry Khan Academy.
From www.khanacademy.org
Acidbase titrations (practice) Khan Academy Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. This unit is part of the chemistry library. In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website.. Titration Introduction Chemistry Khan Academy.
From www.studypool.com
SOLUTION Acid base titration curves titrations chemical processes mcat Titration Introduction Chemistry Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. Browse videos, articles, and exercises by topic. This unit is part of the chemistry library. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).. Titration Introduction Chemistry Khan Academy.
From www.studypool.com
SOLUTION Acid base titration curves titrations chemical processes mcat Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. This unit is part of the chemistry library. If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Buffer capacity Buffers, titrations, and solubility equilibria Titration Introduction Chemistry Khan Academy Browse videos, articles, and exercises by topic. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're behind a web filter, please. If you're seeing this message, it means we're. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Acid base titration example Chemistry Khan Academy YouTube Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known concentration (the titrant) is added to a solution. Titration Introduction Chemistry Khan Academy.
From hxectggyv.blob.core.windows.net
Back Titration Khan Academy at Elizabeth Walker blog Titration Introduction Chemistry Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. This unit is part of the chemistry library. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.scribd.com
Titration Curves & Equivalence Point (Article) Khan Academy PDF Titration Introduction Chemistry Khan Academy In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).. Titration Introduction Chemistry Khan Academy.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Introduction Chemistry Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. In a titration, a solution of. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're seeing this message, it means we're having trouble loading external resources on. Titration Introduction Chemistry Khan Academy.
From www.tes.com
KS3 GCSE Chemistry Introduction To Titrations Lesson Presentation and Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. This unit is part of the chemistry library. If you're seeing this message, it means we're having trouble loading external resources on our website. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration experiment, a 0.020 m. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Weak basestrong acid titrations Acids and bases AP Chemistry Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having trouble loading external resources on our website.. Titration Introduction Chemistry Khan Academy.
From www.tes.com
Introduction To Titration AP GSCE IB Basic To Advance Chemistry Lesson Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titrations of polyprotic acids Acids and bases AP Chemistry Khan Titration Introduction Chemistry Khan Academy In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Strong acidstrong base titrations Acids and bases AP Chemistry Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a. Titration Introduction Chemistry Khan Academy.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). This unit is part of the chemistry library. Browse videos, articles, and exercises by topic. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Acid base titration example Chemistry Khan Academy YouTube Titration Introduction Chemistry Khan Academy If you're behind a web filter, please. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're seeing this message, it means we're having. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Introduction to Titration YouTube Titration Introduction Chemistry Khan Academy This unit is part of the chemistry library. In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. Browse videos, articles, and exercises by topic. In a titration, a solution of known. Titration Introduction Chemistry Khan Academy.
From www.youtube.com
Titration of a weak base with a strong acid (continued) Khan Academy Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Browse videos, articles, and exercises by topic. This unit is part of the chemistry library. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If. Titration Introduction Chemistry Khan Academy.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004 Titration Introduction Chemistry Khan Academy In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website. This unit is part of the chemistry library. Browse videos, articles, and exercises by topic.. Titration Introduction Chemistry Khan Academy.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Introduction Chemistry Khan Academy In a titration experiment, a 0.020 m solution of kmno a 4 (a q) is added from a buret to an acidified sample of h a 2 o a 2 (a q) in a flask. If you're seeing this message, it means we're having trouble loading external resources on our website. Browse videos, articles, and exercises by. Titration Introduction Chemistry Khan Academy.