Halogenation Bromine Radical . radical halogenation of alkanes. We’ll go over the intricacies of the mechanism, how to find. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). What does it mean a selective halogenation and. in this tutorial, we are going to talk about the radical halogenation of alkanes. This reaction is a poor synthetic method due to the formation of polyhalogenated side products. So, two main questions we will address in this post: An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). it can be achieved by a selective halogenation using bromine. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages:
from www.organicchemistrytutor.com
We’ll go over the intricacies of the mechanism, how to find. radical halogenation of alkanes. in this tutorial, we are going to talk about the radical halogenation of alkanes. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: This reaction is a poor synthetic method due to the formation of polyhalogenated side products. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). it can be achieved by a selective halogenation using bromine.
Radical Halogenation of Alkanes — Organic Chemistry Tutor
Halogenation Bromine Radical radical halogenation of alkanes. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). it can be achieved by a selective halogenation using bromine. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). This reaction is a poor synthetic method due to the formation of polyhalogenated side products. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. radical halogenation of alkanes. in this tutorial, we are going to talk about the radical halogenation of alkanes. What does it mean a selective halogenation and. We’ll go over the intricacies of the mechanism, how to find. So, two main questions we will address in this post:
From www.transformationtutoring.com
A Complete Guide To Radical Reactions Halogenation Bromine Radical compounds other than chlorine and methane go through halogenation with the radical chain mechanism. in this tutorial, we are going to talk about the radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: An finally, two methyl radicals can couple to form a side. Halogenation Bromine Radical.
From www.slideserve.com
PPT Free Radical Reactions Halogenation of Alkanes PowerPoint Halogenation Bromine Radical compounds other than chlorine and methane go through halogenation with the radical chain mechanism. radical halogenation of alkanes. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). So, two main questions we will address in this post: the bromination mechanism is the same as for any other free. Halogenation Bromine Radical.
From www.numerade.com
SOLVED The freeradical bromination of the following compound Halogenation Bromine Radical We’ll go over the intricacies of the mechanism, how to find. So, two main questions we will address in this post: in this tutorial, we are going to talk about the radical halogenation of alkanes. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. . Halogenation Bromine Radical.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Halogenation Bromine Radical So, two main questions we will address in this post: halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. in this tutorial, we are going to talk about the radical halogenation of alkanes. the bromination mechanism is the same as for any other free. Halogenation Bromine Radical.
From www.coursehero.com
[Solved] Radical bromination of Menthene gives only two products Halogenation Bromine Radical a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). radical halogenation of alkanes. it can be achieved by a selective halogenation using bromine. So, two main questions we will address in this post: What does it mean a selective halogenation and. in this tutorial, we are going to talk. Halogenation Bromine Radical.
From www.masterorganicchemistry.com
What is Allylic Bromination? Master Organic Chemistry Halogenation Bromine Radical What does it mean a selective halogenation and. in this tutorial, we are going to talk about the radical halogenation of alkanes. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. a chlorine radical and a methyl radical can couple to form more product. Halogenation Bromine Radical.
From quizlet.com
What is the stereochemical of radical bromination of Quizlet Halogenation Bromine Radical halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. it can be achieved by a selective halogenation using bromine. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: a chlorine radical and a methyl. Halogenation Bromine Radical.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Halogenation Bromine Radical it can be achieved by a selective halogenation using bromine. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). radical halogenation of alkanes. What does it mean a selective halogenation and. We’ll go over the intricacies of the mechanism, how to find. An finally, two methyl radicals can couple to. Halogenation Bromine Radical.
From www.masterorganicchemistry.com
Free Radical Halogenation of Alkanes Introduction — Master Organic Halogenation Bromine Radical radical halogenation of alkanes. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). it can be achieved by a selective halogenation using bromine. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). What does it mean a selective halogenation and. This. Halogenation Bromine Radical.
From www.youtube.com
AntiMarkovnikov Radical Halogenation of Alkenes YouTube Halogenation Bromine Radical This reaction is a poor synthetic method due to the formation of polyhalogenated side products. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: We’ll go over the intricacies of the mechanism, how to find. it can be achieved by a selective halogenation using bromine. So, two main questions. Halogenation Bromine Radical.
From www.organicchemistrytutor.com
Radical Halogenation of Alkanes — Organic Chemistry Tutor Halogenation Bromine Radical the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: What does it mean a selective halogenation and. This reaction is a poor synthetic method due to the formation of polyhalogenated side products. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). . Halogenation Bromine Radical.
From sites.science.oregonstate.edu
Reaction Mechanisms Halogenation Bromine Radical What does it mean a selective halogenation and. in this tutorial, we are going to talk about the radical halogenation of alkanes. We’ll go over the intricacies of the mechanism, how to find. it can be achieved by a selective halogenation using bromine. An finally, two methyl radicals can couple to form a side product of ethane (ch. Halogenation Bromine Radical.
From www.studocu.com
Week 4 Notes Free Radical Halogenation, SN2, Nucelophiles Free Halogenation Bromine Radical in this tutorial, we are going to talk about the radical halogenation of alkanes. This reaction is a poor synthetic method due to the formation of polyhalogenated side products. it can be achieved by a selective halogenation using bromine. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. radical halogenation of. Halogenation Bromine Radical.
From www.organicchemistrytutor.com
Radical Halogenation of Alkanes — Organic Chemistry Tutor Halogenation Bromine Radical compounds other than chlorine and methane go through halogenation with the radical chain mechanism. So, two main questions we will address in this post: radical halogenation of alkanes. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). What does it mean a selective halogenation and. halogenation is the reaction. Halogenation Bromine Radical.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Halogenation Bromine Radical the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: radical halogenation of alkanes. So, two main questions we will address in this post: a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). it can be achieved by a selective halogenation. Halogenation Bromine Radical.
From www.slideserve.com
PPT Chapter 12 Radicals Reactions of Alkanes PowerPoint Presentation Halogenation Bromine Radical radical halogenation of alkanes. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). We’ll go over the intricacies of the mechanism, how to find. So, two main questions we will address in this post: What. Halogenation Bromine Radical.
From www.slideserve.com
PPT Radical Chain Mechanism PowerPoint Presentation ID434221 Halogenation Bromine Radical it can be achieved by a selective halogenation using bromine. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). in this tutorial, we are going to talk about the radical halogenation of alkanes. What does it mean a selective halogenation and. the bromination mechanism is the same as. Halogenation Bromine Radical.
From www.chemistrysteps.com
Selectivity in Radical Halogenation with Practice Problems Chemistry Halogenation Bromine Radical So, two main questions we will address in this post: the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: in this tutorial, we are going to talk about the radical halogenation of alkanes. halogenation is the reaction of a halogen with a compound in which a halogen atom. Halogenation Bromine Radical.
From www.docbrown.info
Free radical reaction of alkanes with bromine chlorine conditions Halogenation Bromine Radical radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: We’ll go over the intricacies of the mechanism, how to find. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. This reaction is a poor synthetic method due to the. Halogenation Bromine Radical.
From leah4sci.com
Halogenation of Alkenes Organic Chemistry Reaction Mechanism MCAT Halogenation Bromine Radical This reaction is a poor synthetic method due to the formation of polyhalogenated side products. it can be achieved by a selective halogenation using bromine. What does it mean a selective halogenation and. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: We’ll go over the intricacies of the. Halogenation Bromine Radical.
From www.youtube.com
Radical Bromination of Ethylbenzene in Organic Chemistry YouTube Halogenation Bromine Radical compounds other than chlorine and methane go through halogenation with the radical chain mechanism. So, two main questions we will address in this post: a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). What does it mean a selective halogenation and. We’ll go over the intricacies of the mechanism, how to. Halogenation Bromine Radical.
From www.chemistrysteps.com
Selectivity in Radical Halogenation with Practice Problems Chemistry Halogenation Bromine Radical An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). radical halogenation of alkanes. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. it can be achieved by a selective halogenation using bromine. a chlorine radical and a methyl radical can couple to. Halogenation Bromine Radical.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Halogenation Bromine Radical the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: What does it mean a selective halogenation and. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. So, two main questions we will address in this post: This reaction is a poor synthetic method due. Halogenation Bromine Radical.
From brainly.com
When toluene is used in free radical bromination, a very small amount Halogenation Bromine Radical What does it mean a selective halogenation and. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. it can be achieved by a selective halogenation using bromine. An finally, two. Halogenation Bromine Radical.
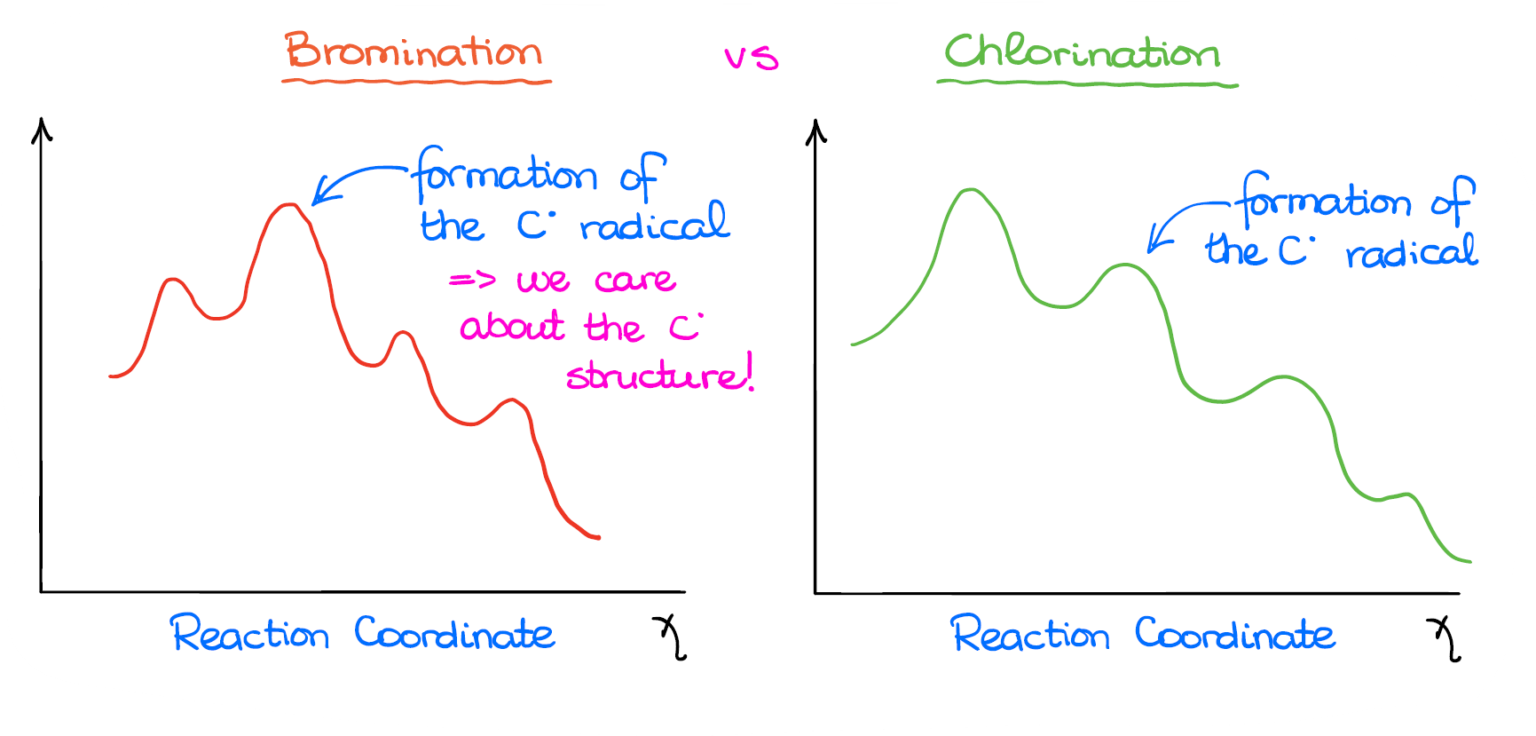
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromination vs. Chlorination Halogenation Bromine Radical So, two main questions we will address in this post: in this tutorial, we are going to talk about the radical halogenation of alkanes. radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: halogenation is the reaction of a halogen with a compound. Halogenation Bromine Radical.
From www.studocu.com
Exp 4radical halogenation procedure Experiment 4 Radical Halogenation Bromine Radical a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). in this tutorial, we are going to talk about the radical halogenation of alkanes. it can be achieved by a selective halogenation using bromine. radical halogenation of alkanes. An finally, two methyl radicals can couple to form a side product. Halogenation Bromine Radical.
From chemistry.stackexchange.com
organic chemistry Products of radical bromination and mechanism Halogenation Bromine Radical What does it mean a selective halogenation and. So, two main questions we will address in this post: in this tutorial, we are going to talk about the radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: radical halogenation of alkanes. a chlorine. Halogenation Bromine Radical.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromination vs. Chlorination Halogenation Bromine Radical radical halogenation of alkanes. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: a chlorine radical and a methyl radical can couple to form more. Halogenation Bromine Radical.
From www.youtube.com
Radical Halogenation of Alkanes YouTube Halogenation Bromine Radical What does it mean a selective halogenation and. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). it can be achieved by a selective halogenation using bromine. radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three. Halogenation Bromine Radical.
From www.numerade.com
SOLVED Question [8] Freeradical bromination of the following compound Halogenation Bromine Radical in this tutorial, we are going to talk about the radical halogenation of alkanes. it can be achieved by a selective halogenation using bromine. compounds other than chlorine and methane go through halogenation with the radical chain mechanism. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). . Halogenation Bromine Radical.
From app.jove.com
Radical Substitution Allylic Bromination Concept Organic Chemistry Halogenation Bromine Radical it can be achieved by a selective halogenation using bromine. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. radical halogenation of alkanes. the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: This reaction. Halogenation Bromine Radical.
From www.youtube.com
Radical Bromination The Primary Alkane Reaction (Theory & Practice Halogenation Bromine Radical We’ll go over the intricacies of the mechanism, how to find. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. a chlorine radical and a methyl radical can couple to form more product (ch 3 cl). An finally, two methyl radicals can couple to form. Halogenation Bromine Radical.
From chemistry.stackexchange.com
organic chemistry Products of radical bromination and mechanism Halogenation Bromine Radical radical halogenation of alkanes. What does it mean a selective halogenation and. in this tutorial, we are going to talk about the radical halogenation of alkanes. halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance. a chlorine radical and a methyl radical can. Halogenation Bromine Radical.
From www.chegg.com
Solved Free radical bromination of an alkane with a halogen Halogenation Bromine Radical This reaction is a poor synthetic method due to the formation of polyhalogenated side products. An finally, two methyl radicals can couple to form a side product of ethane (ch 3 ch 3). So, two main questions we will address in this post: radical halogenation of alkanes. compounds other than chlorine and methane go through halogenation with the. Halogenation Bromine Radical.
From www.chemistrysteps.com
Selectivity in Radical Halogenation with Practice Problems Chemistry Halogenation Bromine Radical the bromination mechanism is the same as for any other free radical halogenation and consists of three stages: in this tutorial, we are going to talk about the radical halogenation of alkanes. This reaction is a poor synthetic method due to the formation of polyhalogenated side products. We’ll go over the intricacies of the mechanism, how to find.. Halogenation Bromine Radical.