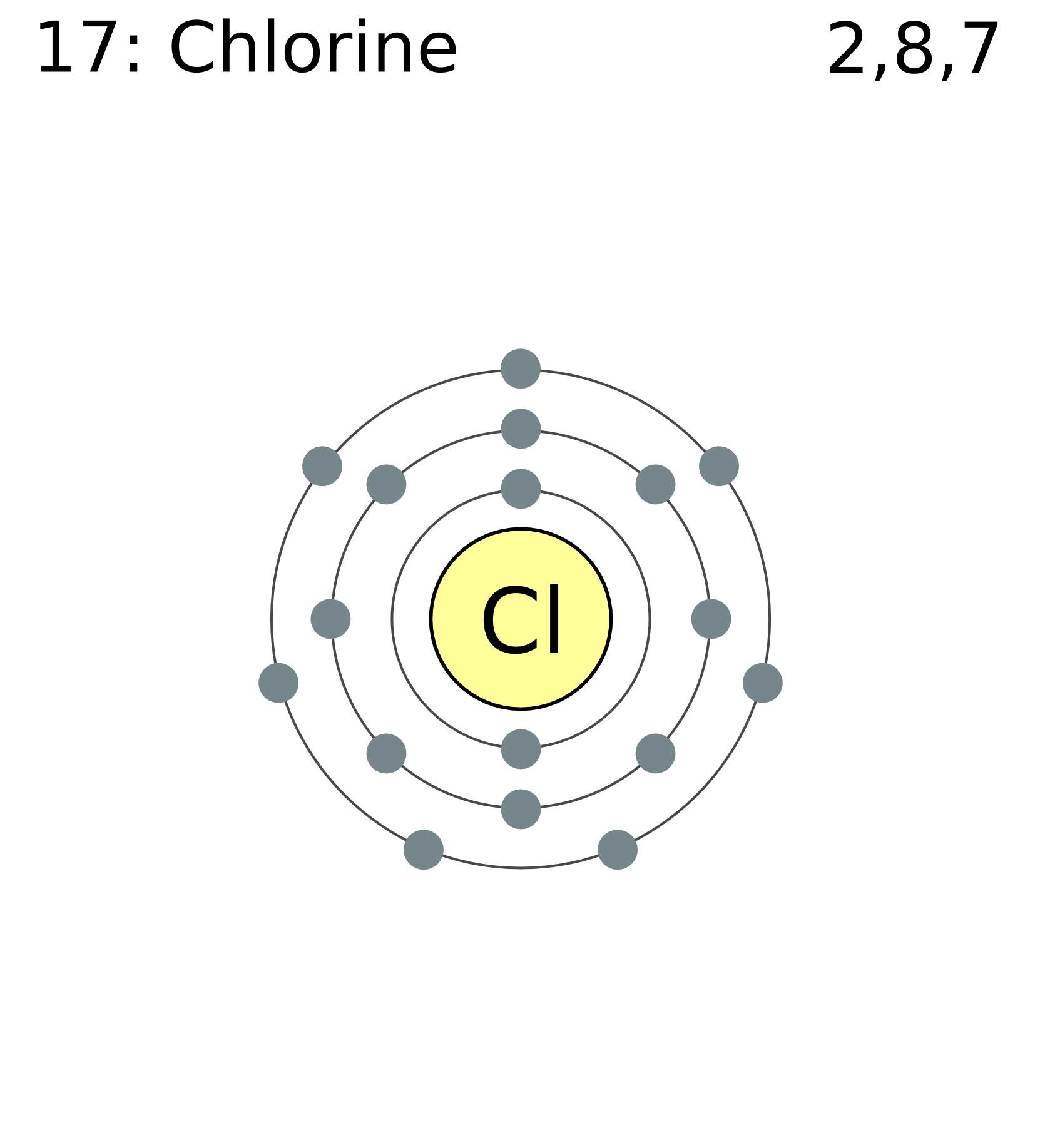
Excited Chlorine Atom Electron Configuration . Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Boron (atomic number 5) has five electrons. Chlorine, a halogen, is crucial in many chemical. The electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17,. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Full ground state electron configuration: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Four electrons fill both the 1s and 2s orbitals. Chlorine has 17 electrons in total. The fifth electron is added to a 2p orbital, the sublevel next higher in. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1.
from commons.wikimedia.org
The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. The electron configuration of chlorine is [ne] 3s2 3p5. The fifth electron is added to a 2p orbital, the sublevel next higher in. Chlorine has 17 electrons in total. Chlorine has an atomic number of 17,. Chlorine, a halogen, is crucial in many chemical. Full ground state electron configuration: Four electrons fill both the 1s and 2s orbitals. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
FileElectron shell 017 chlorine.png Wikimedia Commons
Excited Chlorine Atom Electron Configuration Boron (atomic number 5) has five electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine has 17 electrons in total. Chlorine, a halogen, is crucial in many chemical. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. The electron configuration of chlorine is [ne] 3s2 3p5. Four electrons fill both the 1s and 2s orbitals. Boron (atomic number 5) has five electrons. Full ground state electron configuration: Chlorine has an atomic number of 17,. The fifth electron is added to a 2p orbital, the sublevel next higher in. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Excited Chlorine Atom Electron Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine, a halogen, is crucial in many chemical. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine has an atomic number of. Excited Chlorine Atom Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Excited Chlorine Atom Electron Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. Full ground state electron configuration: Chlorine has 17 electrons in total. Boron (atomic number 5) has five electrons. The fifth electron is added to a 2p. Excited Chlorine Atom Electron Configuration.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Excited Chlorine Atom Electron Configuration The fifth electron is added to a 2p orbital, the sublevel next higher in. Four electrons fill both the 1s and 2s orbitals. The electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17,. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Determine the electronic configuration. Excited Chlorine Atom Electron Configuration.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Excited Chlorine Atom Electron Configuration Four electrons fill both the 1s and 2s orbitals. Chlorine, a halogen, is crucial in many chemical. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The. Excited Chlorine Atom Electron Configuration.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Excited Chlorine Atom Electron Configuration Boron (atomic number 5) has five electrons. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Four electrons fill both the 1s and 2s orbitals. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are. Excited Chlorine Atom Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Excited Chlorine Atom Electron Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Boron (atomic number 5) has five electrons. Full ground state electron configuration: Four electrons fill both the 1s and 2s orbitals. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2. Excited Chlorine Atom Electron Configuration.
From sciencenotes.org
Chlorine Facts Excited Chlorine Atom Electron Configuration Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Four electrons fill both the 1s and 2s orbitals. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. Full ground state electron configuration: Chlorine has an atomic number of 17,. Chlorine. Excited Chlorine Atom Electron Configuration.
From www.toppr.com
Chlorine atom in its third excited state with fluo Excited Chlorine Atom Electron Configuration The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. Chlorine has 17 electrons in total. Boron (atomic number 5) has five electrons. The electron configuration of chlorine is [ne] 3s2 3p5. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital.. Excited Chlorine Atom Electron Configuration.
From www.coursehero.com
[Solved] Identify the neutral element represented by this excited‑state Excited Chlorine Atom Electron Configuration Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Four electrons fill both the 1s and 2s orbitals. Chlorine has 17 electrons in total. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The electron configuration of chlorine is [ne]. Excited Chlorine Atom Electron Configuration.
From diagramtabormanages.z21.web.core.windows.net
Diagram Of Chlorine Excited Chlorine Atom Electron Configuration Full ground state electron configuration: Four electrons fill both the 1s and 2s orbitals. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. Chlorine, a halogen, is crucial in many chemical. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom. Excited Chlorine Atom Electron Configuration.
From pixels.com
Chlorine Electron Configuration Photograph by Excited Chlorine Atom Electron Configuration Full ground state electron configuration: Boron (atomic number 5) has five electrons. Chlorine has 17 electrons in total. Chlorine, a halogen, is crucial in many chemical. Four electrons fill both the 1s and 2s orbitals. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. The fifth electron is. Excited Chlorine Atom Electron Configuration.
From www.slideserve.com
PPT 3 rd Orbitals PowerPoint Presentation, free download ID2062267 Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. Chlorine has an atomic number of 17,. Full ground state electron configuration: Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. The fifth electron is added to a 2p orbital, the sublevel next higher in. Find the full electronic configuration. Excited Chlorine Atom Electron Configuration.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine has an atomic number of 17,. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17. Excited Chlorine Atom Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. Chlorine has an atomic number of 17,. Full ground state electron configuration: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electronic configuration for a chlorine atom that has one electron excited from the. Excited Chlorine Atom Electron Configuration.
From www.youtube.com
Chlorine Electron Configuration YouTube Excited Chlorine Atom Electron Configuration Boron (atomic number 5) has five electrons. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine has an atomic number of 17,. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons).. Excited Chlorine Atom Electron Configuration.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Excited Chlorine Atom Electron Configuration The fifth electron is added to a 2p orbital, the sublevel next higher in. The electron configuration of chlorine is [ne] 3s2 3p5. Four electrons fill both the 1s and 2s orbitals. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Full ground state electron configuration: The excited. Excited Chlorine Atom Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine has an atomic number of. Excited Chlorine Atom Electron Configuration.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Excited Chlorine Atom Electron Configuration The electron configuration of chlorine is [ne] 3s2 3p5. Boron (atomic number 5) has five electrons. Chlorine has an atomic number of 17,. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. The fifth electron is added to a 2p orbital, the sublevel next higher in. Find the. Excited Chlorine Atom Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Excited Chlorine Atom Electron Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The fifth electron is added to a 2p orbital, the sublevel next higher in. Four electrons fill both the 1s and 2s orbitals. Find the full electronic configuration and valence electrons of any periodic element. Excited Chlorine Atom Electron Configuration.
From valenceelectrons.com
How to Write the Electron Configuration for Chlorine (Cl)? Excited Chlorine Atom Electron Configuration Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine has 17 electrons in total. Boron (atomic number 5) has five electrons. The fifth electron is added to a 2p orbital, the sublevel next higher in. Chlorine, a halogen, is crucial in many chemical. Chlorine has an atomic. Excited Chlorine Atom Electron Configuration.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Excited Chlorine Atom Electron Configuration Chlorine has an atomic number of 17,. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Boron (atomic number 5) has five electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Four electrons fill both. Excited Chlorine Atom Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Excited Chlorine Atom Electron Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Four electrons fill both the 1s and 2s orbitals. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Boron (atomic number 5) has five electrons. The excited state electronic configuration of. Excited Chlorine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Excited Chlorine Atom Electron Configuration The fifth electron is added to a 2p orbital, the sublevel next higher in. Four electrons fill both the 1s and 2s orbitals. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. Boron (atomic number 5) has five electrons. Determine the electronic configuration for a chlorine atom that has one electron excited. Excited Chlorine Atom Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Excited Chlorine Atom Electron Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Chlorine has 17 electrons in total. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1. Excited Chlorine Atom Electron Configuration.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Excited Chlorine Atom Electron Configuration Boron (atomic number 5) has five electrons. Four electrons fill both the 1s and 2s orbitals. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. The electron. Excited Chlorine Atom Electron Configuration.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. The electron configuration of chlorine is [ne] 3s2 3p5. Full ground state electron configuration: The fifth electron is added to a 2p orbital, the sublevel next higher in. Four electrons fill both the 1s and. Excited Chlorine Atom Electron Configuration.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Excited Chlorine Atom Electron Configuration Chlorine, a halogen, is crucial in many chemical. The fifth electron is added to a 2p orbital, the sublevel next higher in. Full ground state electron configuration: Four electrons fill both the 1s and 2s orbitals. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17. Excited Chlorine Atom Electron Configuration.
From www.slideshare.net
Atomic concepts Excited Chlorine Atom Electron Configuration The fifth electron is added to a 2p orbital, the sublevel next higher in. Chlorine, a halogen, is crucial in many chemical. Four electrons fill both the 1s and 2s orbitals. The electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has 17 electrons in total. In order to write the chlorine electron configuration we first need to know the. Excited Chlorine Atom Electron Configuration.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Excited Chlorine Atom Electron Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The fifth electron is added to a 2p orbital, the sublevel next higher in. Boron (atomic number 5) has five electrons. Full ground state electron configuration: Chlorine, a halogen, is crucial in many chemical. Four electrons fill both the 1s and 2s orbitals.. Excited Chlorine Atom Electron Configuration.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Excited Chlorine Atom Electron Configuration Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Boron (atomic number 5) has five electrons. Chlorine has an atomic number of 17,. Chlorine, a halogen, is crucial in many chemical. Chlorine has 17 electrons in total. The excited state electronic configuration of the cl is 1s2 2s2. Excited Chlorine Atom Electron Configuration.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Excited Chlorine Atom Electron Configuration Boron (atomic number 5) has five electrons. Full ground state electron configuration: The electron configuration of chlorine is [ne] 3s2 3p5. Four electrons fill both the 1s and 2s orbitals. The fifth electron is added to a 2p orbital, the sublevel next higher in. In order to write the chlorine electron configuration we first need to know the number of. Excited Chlorine Atom Electron Configuration.
From www.slideserve.com
PPT The University of the State of New York REGENTS HIGH SCHOOL Excited Chlorine Atom Electron Configuration Four electrons fill both the 1s and 2s orbitals. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine has an atomic number of 17,. Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the. Excited Chlorine Atom Electron Configuration.
From byjus.com
ntMaximum value (n+l+m) for unpaired electrons in second excited state Excited Chlorine Atom Electron Configuration Determine the electronic configuration for a chlorine atom that has one electron excited from the 3p orbital to the 4s orbital. Chlorine, a halogen, is crucial in many chemical. Four electrons fill both the 1s and 2s orbitals. Full ground state electron configuration: Chlorine has 17 electrons in total. Find the full electronic configuration and valence electrons of any periodic. Excited Chlorine Atom Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Excited Chlorine Atom Electron Configuration Chlorine has an atomic number of 17,. The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: Chlorine has 17 electrons in total.. Excited Chlorine Atom Electron Configuration.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Excited Chlorine Atom Electron Configuration Full ground state electron configuration: The excited state electronic configuration of the cl is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3dxy1. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electronic configuration for a chlorine atom that has one electron excited. Excited Chlorine Atom Electron Configuration.