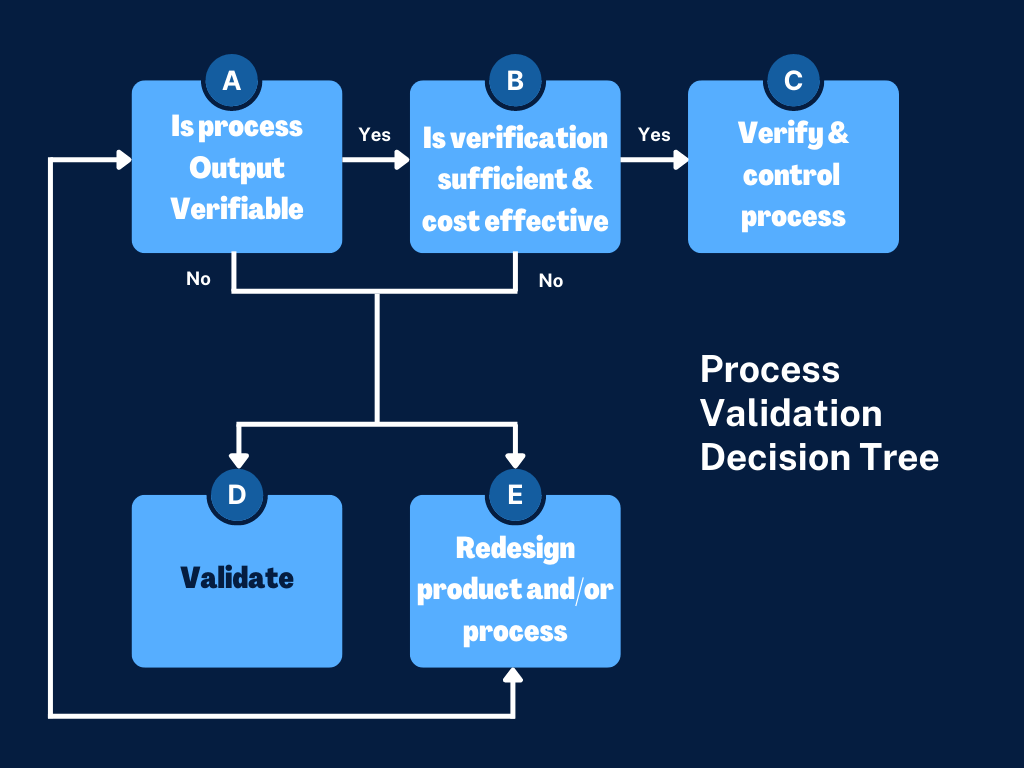
Manufacturing Validation Process . Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. In this comprehensive guide, we will. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is.
from fasttrackiso13485.com
This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.
Fast Track ISO 13485 Process Validation Explained for your Medical Device
Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the.
From www.researchgate.net
Flowchart of manufacturing process validation Download Scientific Diagram Manufacturing Validation Process Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. In this comprehensive. Manufacturing Validation Process.
From kvalito.ch
How is a system validated? Kvalito Manufacturing Validation Process Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a. Manufacturing Validation Process.
From guideline-sop.com
Process Validation (PV) & Verification of Drug Product Guidelines SOPs Manufacturing Validation Process Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. This. Manufacturing Validation Process.
From econsulting.co
Validation of Manufacturing Process eConsulting Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and. Manufacturing Validation Process.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Manufacturing Validation Process In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Process validation means establishing by objective evidence that a process consistently produces a result or product. Manufacturing Validation Process.
From www.slideshare.net
PROCESS VALIDATION Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. This. Manufacturing Validation Process.
From www.slideserve.com
PPT PROCESS VALIDATION OF OINTMENT/CREAM FORMULATION PowerPoint Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and. Manufacturing Validation Process.
From www.youtube.com
Process Validation in Pharmaceutical Manufacturing Validation in Manufacturing Validation Process This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Good manufacturing practices (gmp). Manufacturing Validation Process.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Manufacturing Validation Process In this comprehensive guide, we will. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and validation (or v&v) are two separate but related processes that manufacturers use to. Manufacturing Validation Process.
From www.presentationeze.com
Medical Device Manufacturing Validation....PresentationEZE Manufacturing Validation Process Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their. Manufacturing Validation Process.
From www.slideserve.com
PPT MANUFACTURING PROCESS AND VALIDATION PowerPoint Presentation Manufacturing Validation Process In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From www.youtube.com
Manufacturing process validation features YouTube Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and. Manufacturing Validation Process.
From mavenprofserv.com
Versus Validation Understanding Process Verification vs. Validation Manufacturing Validation Process Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. In this comprehensive guide, we will. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From ispe.org
Biopharmaceutical Manufacturing Process Validation and Quality Risk Manufacturing Validation Process In this comprehensive guide, we will. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process. Manufacturing Validation Process.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation is a systematic approach to ensure. Manufacturing Validation Process.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Manufacturing Validation Process Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. In this comprehensive guide, we will. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation. Manufacturing Validation Process.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Manufacturing Validation Process Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a. Manufacturing Validation Process.
From www.scribd.com
Manufacturing Process Validation New PDF Verification And Manufacturing Validation Process This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Verification and validation. Manufacturing Validation Process.
From www.learngxp.com
The Four Types of Process Validation LearnGxP Accredited Online Life Manufacturing Validation Process Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation. Manufacturing Validation Process.
From www.youtube.com
BPV 1 1 Process Validation Workflow YouTube Manufacturing Validation Process Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From cmsmedtech.com
Free ISO 13485 Process Validation Template Manufacturing Validation Process Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Manufacturing Validation Process In this comprehensive guide, we will. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their. Manufacturing Validation Process.
From slcontrols.com
The 3 Stages of Process Validation Explained SL Controls Manufacturing Validation Process In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a. Manufacturing Validation Process.
From www.collidu.com
Process Validation PowerPoint Presentation Slides PPT Template Manufacturing Validation Process In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Manufacturing Validation Process This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Verification and. Manufacturing Validation Process.
From gamma.app
Validation of Equipments and Manufacturing Processes Manufacturing Validation Process This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation is a systematic approach to ensure that. Manufacturing Validation Process.
From www.presentationeze.com
Process Validation versus Process Verification. PresentationEZE Manufacturing Validation Process In this comprehensive guide, we will. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure. Manufacturing Validation Process.
From www.pngegg.com
Validation master plan Verification and validation Process validation Manufacturing Validation Process Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Good manufacturing. Manufacturing Validation Process.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Manufacturing Validation Process Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. In this comprehensive guide, we will. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From www.slideserve.com
PPT Process Validation General Principles and Practices PowerPoint Manufacturing Validation Process In this comprehensive guide, we will. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process validation means. Manufacturing Validation Process.
From www.slideserve.com
PPT MANUFACTURING PROCESS AND VALIDATION PowerPoint Presentation Manufacturing Validation Process Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Good manufacturing practices. Manufacturing Validation Process.
From orioledhub.eu
The Lifecycle Approach to Process Validation Overview Orioled Hub Manufacturing Validation Process In this comprehensive guide, we will. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Process. Manufacturing Validation Process.
From www.slideshare.net
Process validation Manufacturing Validation Process Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation is a systematic approach to ensure that a manufacturing process consistently produces a product of predetermined quality. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. This guidance outlines the general principles and approaches. Manufacturing Validation Process.
From bonezonepub.com
Understanding Process Validation Requirements for Orthopedic Products Manufacturing Validation Process Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. In this comprehensive guide, we will. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Manufacturing Validation Process.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Manufacturing Validation Process This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. In this comprehensive guide, we will. Verification and validation (or v&v) are two separate but related processes that manufacturers use to ensure their product is. Good manufacturing practices (gmp) validation is a systematic approach that involves establishing documented evidence. Process validation is. Manufacturing Validation Process.