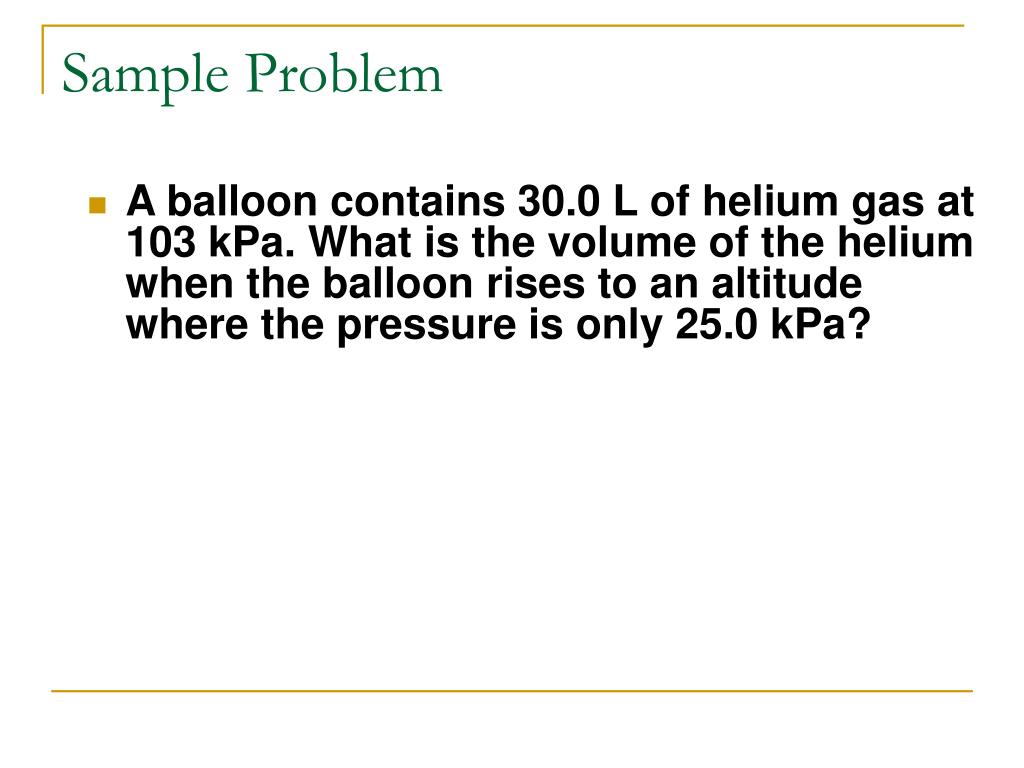
Unit 9 Gas Laws Review Answers . If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. Consequently, the collisions of the gas molecules with the walls of the container occur more. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. The ideal gas law reflects how gases are supposed to behave, and often do behave. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. Increasing the temperature of a gas means that the particles have more kinetic energy. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. A gas at stp occupies 28 cm3 of space.
from www.slideserve.com
Increasing the temperature of a gas means that the particles have more kinetic energy. A gas at stp occupies 28 cm3 of space. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. The ideal gas law reflects how gases are supposed to behave, and often do behave. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find.
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free
Unit 9 Gas Laws Review Answers The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Increasing the temperature of a gas means that the particles have more kinetic energy. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. The ideal gas law reflects how gases are supposed to behave, and often do behave. A gas at stp occupies 28 cm3 of space. Consequently, the collisions of the gas molecules with the walls of the container occur more. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. The ideal gas law reflects how gases are supposed to behave, and often do behave. Increasing the temperature of a gas means that the particles have more kinetic energy.. Unit 9 Gas Laws Review Answers.
From studylib.net
Unit 9 The Gas Laws Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. Increasing the temperature of a gas means that the particles have more kinetic energy. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. A gas at stp occupies 28 cm3 of space. Consequently, the collisions. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Consequently, the collisions of the gas molecules with the walls of the container occur more. A gas at stp occupies 28 cm3 of space. Increasing the temperature of a gas means that the particles have more kinetic energy. By joining chemistry steps, you will gain. Unit 9 Gas Laws Review Answers.
From goodimg.co
️Gas Laws Worksheet Chemistry Answers Free Download Goodimg.co Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law,. Unit 9 Gas Laws Review Answers.
From www.chegg.com
Solved Chem 2100 Worksheet 9 Gas Laws Ideal Gas Law PV = Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. Consequently, the collisions of the gas molecules with the walls of. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers A gas at stp occupies 28 cm3 of space. Increasing the temperature of a gas means that the particles have more kinetic energy. Consequently, the collisions of the gas molecules with the walls of the container occur more. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. The _______________ gas law permits calculation. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. Increasing the temperature of a gas means that the particles have. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. Increasing the temperature of a gas means that the particles have more. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Increasing the temperature of a gas means that the. Unit 9 Gas Laws Review Answers.
From www.studocu.com
Phet Simulation Gases Intro Students Unit 9 Gas Laws Name Unit 9 Gas Laws Review Answers The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. Study with quizlet and memorize flashcards containing terms like boyle's law,. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. The ideal gas law reflects how gases are supposed to behave, and often do behave. The _______________ gas law permits calculation of any one term when temperature, pressure, and. Unit 9 Gas Laws Review Answers.
From studylib.net
Gas Laws Worksheet Answer Key Unit 9 Gas Laws Review Answers By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. If the pressure changes to 3.8 atm and the temperature increases to 203oc,. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers Consequently, the collisions of the gas molecules with the walls of the container occur more. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. A gas at stp occupies 28 cm3 of space. Unlike the previous gas laws we. Unit 9 Gas Laws Review Answers.
From www.youtube.com
LM Unit 9 Intro Ideal Gas Law YouTube Unit 9 Gas Laws Review Answers If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. Consequently, the collisions of the gas molecules with the walls of the container occur more. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers Increasing the temperature of a gas means that the particles have more kinetic energy. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2. Unit 9 Gas Laws Review Answers.
From studylib.net
Gas Laws Unit Test ANSWER SHEET Unit 9 Gas Laws Review Answers The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. A gas at stp occupies 28 cm3 of space. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. If. Unit 9 Gas Laws Review Answers.
From lessoncampuselena.z19.web.core.windows.net
Understanding Gas Laws Worksheet Answers Unit 9 Gas Laws Review Answers Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. A gas at stp occupies 28 cm3 of space. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. The ideal gas law reflects how gases are supposed to behave, and often do behave. Consequently, the. Unit 9 Gas Laws Review Answers.
From www.studocu.com
Gas law packet Unit 9 (Chapter 14) Gas Laws Upon completion of Unit Unit 9 Gas Laws Review Answers By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. The initial pressure and volume of a sealed container is inversely proportional to the final. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Increasing the temperature of a gas means that the particles have more kinetic energy. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. A gas at stp occupies 28 cm3 of space. If the pressure changes to 3.8. Unit 9 Gas Laws Review Answers.
From www.chegg.com
Solved Experiment 9 Gas Laws PRELABORATORY ASSIGNMENT This Unit 9 Gas Laws Review Answers Increasing the temperature of a gas means that the particles have more kinetic energy. Unlike the previous gas laws we talked about, this law is about one system that is not undergoing. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Study with quizlet and memorize flashcards containing terms like boyle's. Unit 9 Gas Laws Review Answers.
From studylib.net
Gas Laws Test Review Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Consequently, the collisions of the gas molecules with the walls of the container occur more. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. Study with quizlet and memorize flashcards containing terms like boyle's law,. Unit 9 Gas Laws Review Answers.
From www.chegg.com
Solved Name ] Worksheet 9 Gas Laws Directions Answer each Unit 9 Gas Laws Review Answers A gas at stp occupies 28 cm3 of space. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. If the pressure changes to 3.8 atm and the temperature increases to 203oc, find. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. A gas at stp occupies 28 cm3 of space.. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers A gas at stp occupies 28 cm3 of space. The ideal gas law reflects how gases are supposed to behave, and often do behave. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. By. Unit 9 Gas Laws Review Answers.
From www.slideserve.com
PPT Chemistry Unit 9 Gas Laws PowerPoint Presentation, free Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. A gas at stp occupies 28 cm3 of space. Increasing the temperature of a gas means that the particles have more kinetic energy. The ideal gas law reflects how gases are supposed to behave, and often do behave. Consequently, the collisions of the. Unit 9 Gas Laws Review Answers.
From www.pdffiller.com
Fillable Online Unit 9 Gas Laws Worksheets 1 6S2016 Fax Email Print Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. The initial pressure and volume of a sealed container is inversely proportional. Unit 9 Gas Laws Review Answers.
From www.youtube.com
LM Unit 9 Gas Law Intro YouTube Unit 9 Gas Laws Review Answers Increasing the temperature of a gas means that the particles have more kinetic energy. The ideal gas law reflects how gases are supposed to behave, and often do behave. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. The initial pressure and volume of a sealed container is inversely proportional. Unit 9 Gas Laws Review Answers.
From www.chegg.com
Solved Worksheet 9 Gas Laws Directions Answer cach of the Unit 9 Gas Laws Review Answers Consequently, the collisions of the gas molecules with the walls of the container occur more. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. The ideal gas law reflects how gases are supposed to. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers The ideal gas law reflects how gases are supposed to behave, and often do behave. Increasing the temperature of a gas means that the particles have more kinetic energy. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers Study with quizlet and memorize flashcards containing terms like boyle's law, vapor pressure, diffusion and more. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. The _______________ gas law permits calculation of any one term when temperature, pressure, and volume change for a gas. A gas at stp occupies 28 cm3. Unit 9 Gas Laws Review Answers.
From slideplayer.com
Unit 9 Gas Laws. ppt download Unit 9 Gas Laws Review Answers By joining chemistry steps, you will gain instant access to the answers and solutions for all the practice problems, quizzes, and the powerful set of general chemistry 1 and 2 summary. A gas at stp occupies 28 cm3 of space. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. Increasing the temperature. Unit 9 Gas Laws Review Answers.
From www.studocu.com
Gas law questions Notes HS/Science Unit 09 Lesson 01 More Unit 9 Gas Laws Review Answers Consequently, the collisions of the gas molecules with the walls of the container occur more. Study with quizlet and memorize flashcards containing terms like dalton's law, boyle's law, charle's law and more. A gas at stp occupies 28 cm3 of space. The initial pressure and volume of a sealed container is inversely proportional to the final pressure and volume. By. Unit 9 Gas Laws Review Answers.