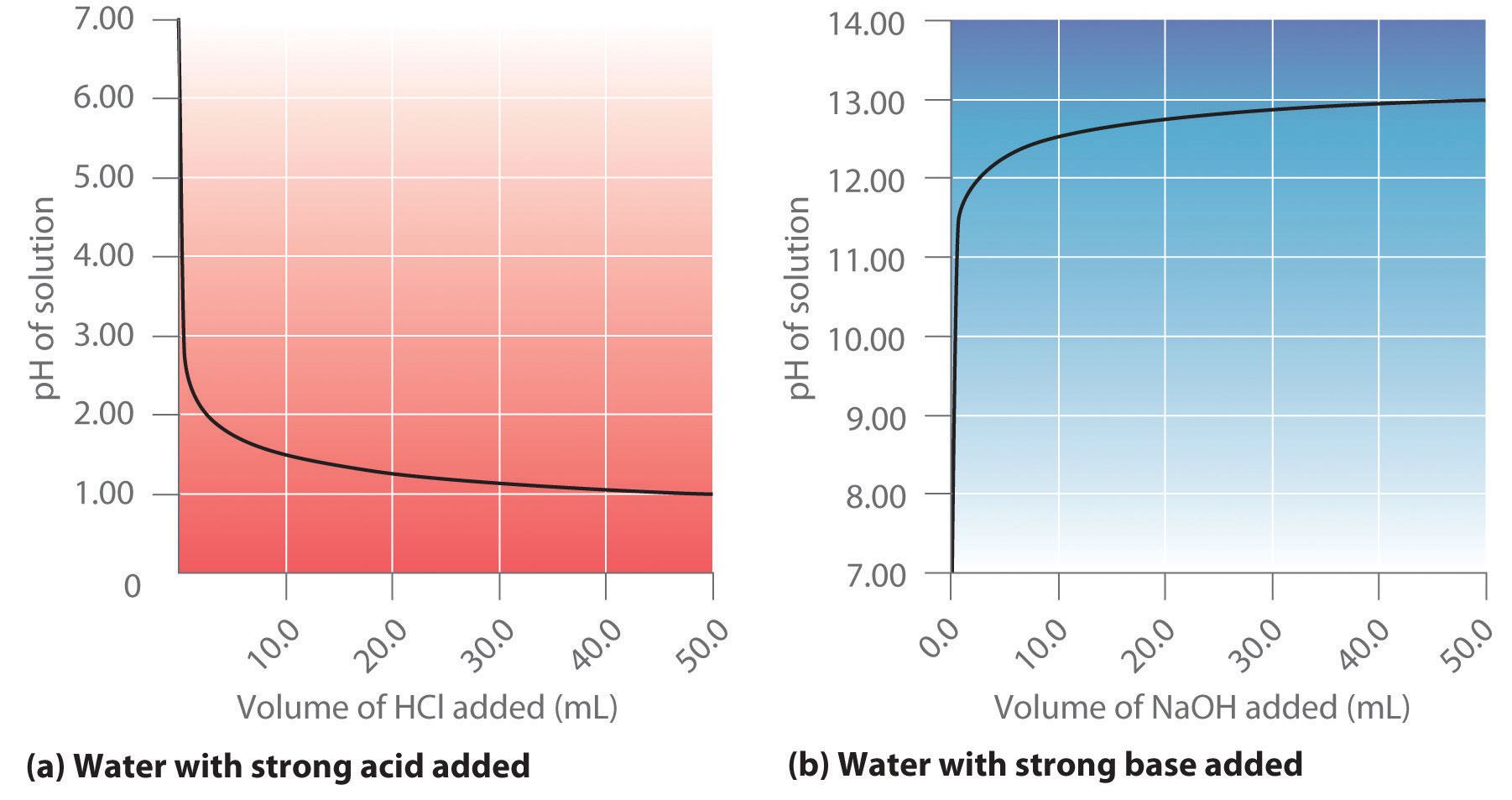
Titration Table . Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find out how to record, process and.
from
Find out how to record, process and. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about.
Titration Table A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Find a table of common acids and bases and their strengths, and faqs about. Find out how to record, process and. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.
From
Titration Table Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Find out how to record, process and. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of. Titration Table.
From www.slideshare.net
Titrations Titration Table A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Learn how to measure the concentration of a substance in. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find out how to record, process and. Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Learn how to measure the. Titration Table.
From
Titration Table A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding. Titration Table.
From
Titration Table Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Find out how to record, process and. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a technique used. Titration Table.
From
Titration Table The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Learn how to perform a titration. Titration Table.
From www.researchgate.net
Titration data for the emission properties (at 420 nm) of the four... Download Table Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The example below demonstrates the technique to solve a titration. Titration Table.
From
Titration Table Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about. The example below demonstrates the technique to solve a titration problem for. Titration Table.
From www.researchgate.net
Screening results of various titrations Download Table Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Find out how to record, process and. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Learn how to measure. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a technique used in. Titration Table.
From
Titration Table The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find out how to record, process and. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to perform a titration and volumetric analysis with a burette,. Titration Table.
From mavink.com
Levophed Titration Chart Titration Table Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The example below demonstrates the. Titration Table.
From
Titration Table Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find a table of common acids and bases and their strengths, and faqs. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find out how to record, process and. Learn how to measure the. Titration Table.
From
Titration Table The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding. Titration Table.
From
Titration Table Find out how to record, process and. Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte.. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find a table of common acids and bases and their strengths, and faqs about. Find out how to record, process and.. Titration Table.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 Titration Table Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an. Titration Table.
From
Titration Table Find out how to record, process and. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find a table of common acids. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. The example below demonstrates the technique to solve a titration. Titration Table.
From
Titration Table Find out how to record, process and. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the. Titration Table.
From www.chegg.com
table 1 Preparation of solutions before titration Titration Table Find out how to record, process and. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find a table of common acids and bases and their strengths, and faqs about. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. A titration is a. Titration Table.
From pametno21.blogspot.com
Formula Mass Table pametno Titration Table The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Find a table of common acids and bases and their strengths, and faqs about. Learn how to measure the. Titration Table.
From www.chegg.com
Exercise 1 Back Titration of Antacid Neutralization Titration Table Find a table of common acids and bases and their strengths, and faqs about. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Titration Table.
From
Titration Table Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about.. Titration Table.
From
Titration Table Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find a table of common acids and bases and their strengths, and faqs about. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Find out how to record, process and.. Titration Table.
From
Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Find a table of common acids and bases and their strengths, and faqs about. A titration is a technique used in chemistry to help determine the. Titration Table.
From
Titration Table Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of. Titration Table.
From www.chegg.com
Table 2. Results of titration of EDTA with 0.025 M Titration Table Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Find out how to record, process and. The example below demonstrates the technique to. Titration Table.
From www.chegg.com
Solved Lab Notebook Only complete the table for your Titration Table Titration is a technique to determine the concentration of an unknown solution by adding a known solution of another. Learn how to perform a titration and volumetric analysis with a burette, indicator and pipette. Find a table of common acids and bases and their strengths, and faqs about. The example below demonstrates the technique to solve a titration problem for. Titration Table.