Copper And Hcl Balanced Equation . to be useful, chemical equations must always be balanced. This is a requirement the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. the net ionic equation is. The coefficients in a balanced equation must. Balanced chemical equations have the same number and type of each atom on both sides of the equation. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). Cu + 2 hcl → cu (cl) 2 + h 2. 2 hcl + cu → cucl 2 + h 2. in this video we'll look at the chemical equation for the reaction of hcl. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: First, we balance the molecular equation.
from ar.inspiredpencil.com
the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. First, we balance the molecular equation. Cu + 2 hcl → cu (cl) 2 + h 2. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: 2 hcl + cu → cucl 2 + h 2. Balanced chemical equations have the same number and type of each atom on both sides of the equation. in this video we'll look at the chemical equation for the reaction of hcl. to be useful, chemical equations must always be balanced. the net ionic equation is. The coefficients in a balanced equation must.
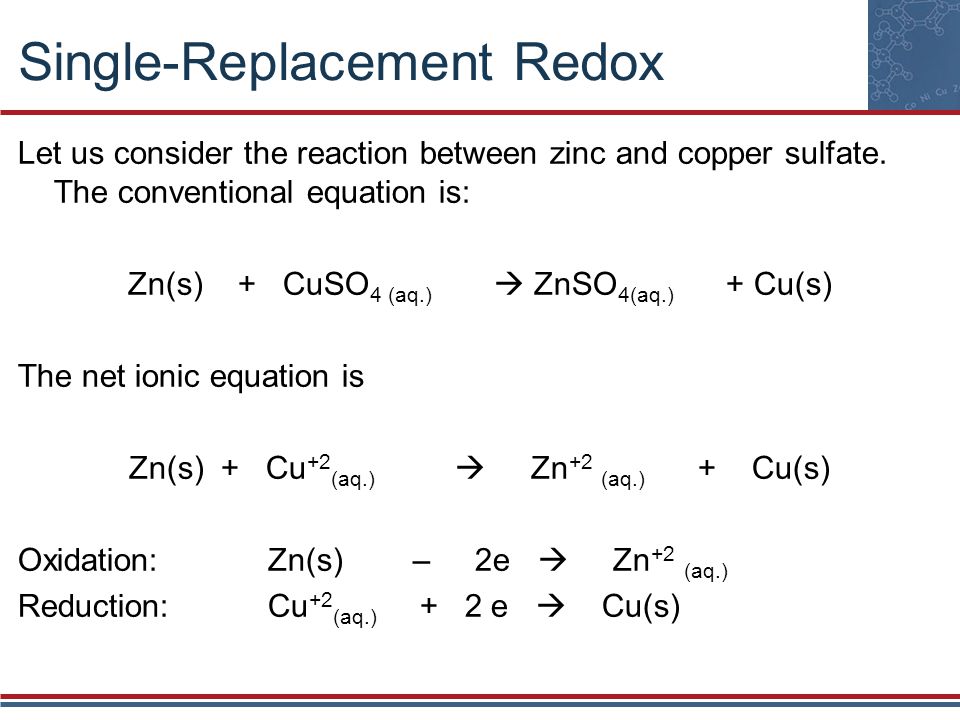
Single Replacement Reaction Equation
Copper And Hcl Balanced Equation 2 hcl + cu → cucl 2 + h 2. 2 hcl + cu → cucl 2 + h 2. in this video we'll look at the chemical equation for the reaction of hcl. This is a requirement the. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: Balanced chemical equations have the same number and type of each atom on both sides of the equation. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. The coefficients in a balanced equation must. First, we balance the molecular equation. the net ionic equation is. to be useful, chemical equations must always be balanced. Cu + 2 hcl → cu (cl) 2 + h 2. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid).
From brainly.in
Balance the chemical equation Mg(OH)2 + HCl _ MgCl2 + H2O Brainly.in Copper And Hcl Balanced Equation in this video we'll look at the chemical equation for the reaction of hcl. Cu + 2 hcl → cu (cl) 2 + h 2. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. First, we balance. Copper And Hcl Balanced Equation.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper And Hcl Balanced Equation Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: to be useful, chemical equations must always be balanced. Cu + 2 hcl → cu (cl) 2 + h 2. in this video we'll look at the. Copper And Hcl Balanced Equation.
From www.numerade.com
SOLVED The reaction between solid copper and aqueous silver nitrate Copper And Hcl Balanced Equation This is a requirement the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. to be useful, chemical equations must always be balanced. First, we balance the molecular equation. Cu + 2 hcl → cu (cl) 2. Copper And Hcl Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationCopper Copper And Hcl Balanced Equation Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: to be useful, chemical equations must always be balanced. in this video we'll look at the chemical equation for the reaction of hcl. there are three. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper And Hcl Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Cu + 2 hcl → cu (cl) 2 + h 2. in this video we'll look at the chemical equation for the reaction of hcl. First, we balance. Copper And Hcl Balanced Equation.
From workerdecline11.gitlab.io
Beautiful Work Ammonia And Hydrogen Chloride Gases Are Mixed Equation Copper And Hcl Balanced Equation there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). the net ionic equation is. in this video we'll look at the chemical equation for the reaction of hcl. The coefficients in a balanced equation must. Balanced chemical equations have the same number and type of each atom. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Balance CuO + HCl = CuCl2 + H2O YouTube Copper And Hcl Balanced Equation 2 hcl + cu → cucl 2 + h 2. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). the net ionic equation is. This is a requirement the. to be useful, chemical equations must always be balanced. The coefficients in a balanced equation must. Br −. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper And Hcl Balanced Equation First, we balance the molecular equation. 2 hcl + cu → cucl 2 + h 2. Cu + 2 hcl → cu (cl) 2 + h 2. the net ionic equation is. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the. Copper And Hcl Balanced Equation.
From www.indiamart.com
Powder Copper Gluconate, Non prescription, Packaging Type Packet at Copper And Hcl Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. the net ionic equation is. This is a requirement the. in this video we'll look at the chemical equation for the reaction of hcl. The coefficients in. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Pb(NO3)2 = NaNO3 + Pb(OH Copper And Hcl Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. to be useful, chemical equations must always be balanced. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and. Copper And Hcl Balanced Equation.
From www.chegg.com
Solved Ammonium Hydroxide (NH_4OH) & Hydrochloric Acid (HCI) Copper And Hcl Balanced Equation in this video we'll look at the chemical equation for the reaction of hcl. This is a requirement the. to be useful, chemical equations must always be balanced. Cu + 2 hcl → cu (cl) 2 + h 2. The coefficients in a balanced equation must. there are three main steps for writing the net ionic equation. Copper And Hcl Balanced Equation.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Copper And Hcl Balanced Equation The coefficients in a balanced equation must. the net ionic equation is. 2 hcl + cu → cucl 2 + h 2. in this video we'll look at the chemical equation for the reaction of hcl. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 −. Copper And Hcl Balanced Equation.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper And Hcl Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the. 2 hcl + cu → cucl 2 + h 2. Balanced chemical equations have the same number and type of each atom on both. Copper And Hcl Balanced Equation.
From www.coursehero.com
[Solved] Please balance the following equations, Zinc reacts with Copper And Hcl Balanced Equation there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Br − (aq) + ag + (aq) → agbr(s). Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NH3 + HNO3 = NH4NO3 YouTube Copper And Hcl Balanced Equation Cu + 2 hcl → cu (cl) 2 + h 2. Balanced chemical equations have the same number and type of each atom on both sides of the equation. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). 2 hcl + cu → cucl 2 + h 2. . Copper And Hcl Balanced Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Copper And Hcl Balanced Equation First, we balance the molecular equation. The coefficients in a balanced equation must. the net ionic equation is. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in. Copper And Hcl Balanced Equation.
From www.youtube.com
How to balance H2+Cl2=HClchemical equation H2+Cl2=HClH2+Cl2=HCl Copper And Hcl Balanced Equation This is a requirement the. in this video we'll look at the chemical equation for the reaction of hcl. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. First, we balance the molecular equation. Cu + 2. Copper And Hcl Balanced Equation.
From www.tessshebaylo.com
Balanced Net Ionic Equation For Corrosion Of Iron Tessshebaylo Copper And Hcl Balanced Equation the net ionic equation is. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). The coefficients in a balanced equation must. in this video we'll look at the chemical equation for the reaction of hcl. 2 hcl + cu → cucl 2 + h 2. Br −. Copper And Hcl Balanced Equation.
From treatybottle13.pythonanywhere.com
Beautiful Work Ammonia And Hcl Balanced Equation Nesa Physics Syllabus 2020 Copper And Hcl Balanced Equation in this video we'll look at the chemical equation for the reaction of hcl. to be useful, chemical equations must always be balanced. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Balanced chemical equations have. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Balance H2SO4 + NaHCO3 = Na2SO4 + H2O + CO2 YouTube Copper And Hcl Balanced Equation 2 hcl + cu → cucl 2 + h 2. to be useful, chemical equations must always be balanced. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Cu + 2 hcl → cu (cl) 2 +. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 + HCl = CuCl2 + H2O Copper And Hcl Balanced Equation there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). First, we balance the molecular equation. to be useful, chemical equations must always be balanced. in this video we'll look at the chemical equation for the reaction of hcl. Br − (aq) + ag + (aq) → agbr(s). Copper And Hcl Balanced Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Copper And Hcl Balanced Equation the net ionic equation is. Cu + 2 hcl → cu (cl) 2 + h 2. in this video we'll look at the chemical equation for the reaction of hcl. 2 hcl + cu → cucl 2 + h 2. Balanced chemical equations have the same number and type of each atom on both sides of the equation.. Copper And Hcl Balanced Equation.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Copper And Hcl Balanced Equation Cu + 2 hcl → cu (cl) 2 + h 2. the net ionic equation is. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: in this video we'll look at the chemical equation for the. Copper And Hcl Balanced Equation.
From angnablog.blogspot.com
What Is The Charge Of Copper Ions In Water Solutions Copper And Hcl Balanced Equation Balanced chemical equations have the same number and type of each atom on both sides of the equation. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Copper And Hcl Balanced Equation.
From www.homeworklib.com
Balance the equation, then determine what volume of a 12M HCl solution Copper And Hcl Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. First, we balance the molecular equation. in this video we'll look at the chemical equation for the reaction of hcl. The coefficients in a balanced equation must. . Copper And Hcl Balanced Equation.
From www.youtube.com
Net Ionic Equation for CuCO3 + HCl = H2O + CO2 + CuCl2 YouTube Copper And Hcl Balanced Equation there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). in this video we'll look at the chemical equation for the reaction of hcl. First, we balance the molecular equation. The coefficients in a balanced equation must. Br − (aq) + ag + (aq) → agbr(s) in the second. Copper And Hcl Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for HNO3 + Ca(OH)2 YouTube Copper And Hcl Balanced Equation Cu + 2 hcl → cu (cl) 2 + h 2. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). Balanced chemical equations have the same number and type of each atom on both sides of the equation. the chemical equation described in section 4.1 is balanced, meaning. Copper And Hcl Balanced Equation.
From gruamipz.blogspot.com
Calcium Carbonate Hydrochloric Acid How To Balance Caco3 Hcl Cacl2 Copper And Hcl Balanced Equation The coefficients in a balanced equation must. First, we balance the molecular equation. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. 2 hcl + cu → cucl 2 + h 2. Br − (aq) + ag +. Copper And Hcl Balanced Equation.
From www.science-revision.co.uk
moles and equations Copper And Hcl Balanced Equation in this video we'll look at the chemical equation for the reaction of hcl. Cu + 2 hcl → cu (cl) 2 + h 2. The coefficients in a balanced equation must. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). This is a requirement the. the. Copper And Hcl Balanced Equation.
From www.youtube.com
Cu + HCl (Copper + Hydrochloric acid) Equation YouTube Copper And Hcl Balanced Equation This is a requirement the. the net ionic equation is. Balanced chemical equations have the same number and type of each atom on both sides of the equation. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). Br − (aq) + ag + (aq) → agbr(s) in the. Copper And Hcl Balanced Equation.
From www.chegg.com
Solved Predict the products and write a balanced chemical Copper And Hcl Balanced Equation to be useful, chemical equations must always be balanced. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: in this video we'll look at the chemical equation for the reaction of hcl. there are three. Copper And Hcl Balanced Equation.
From www.youtube.com
Net Ionic Equation for HCl + CaCO3 (See Important Note in Description Copper And Hcl Balanced Equation The coefficients in a balanced equation must. Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: the net ionic equation is. 2 hcl + cu → cucl 2 + h 2. to be useful, chemical equations. Copper And Hcl Balanced Equation.
From ar.inspiredpencil.com
Single Replacement Reaction Equation Copper And Hcl Balanced Equation to be useful, chemical equations must always be balanced. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must. This is a requirement the.. Copper And Hcl Balanced Equation.
From www.youtube.com
Electrolysis half equations for copper chloride YouTube Copper And Hcl Balanced Equation First, we balance the molecular equation. there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). This is a requirement the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and. Copper And Hcl Balanced Equation.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Copper And Hcl Balanced Equation Br − (aq) + ag + (aq) → agbr(s) in the second equation, the mg 2 + (aq) and no 3 − (aq) ions are spectator ions, so they are canceled: there are three main steps for writing the net ionic equation for cu + hcl (copper + hydrochloric acid). First, we balance the molecular equation. in this. Copper And Hcl Balanced Equation.