Cap Definition Of Test System . Test complexities what is “test complexity”? Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Cap laboratory accreditation helps laboratories: Maintain accuracy of test results and ensure accurate patient diagnosis; Clinical laboratory test systems are assigned a moderate or high complexity. Cap believes a laboratory developed. Meet required standards from clia, fda and. Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or.
from www.javatpoint.com
Cap believes a laboratory developed. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap laboratory accreditation helps laboratories: Clinical laboratory test systems are assigned a moderate or high complexity. Test complexities what is “test complexity”? Maintain accuracy of test results and ensure accurate patient diagnosis; Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Meet required standards from clia, fda and. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or.
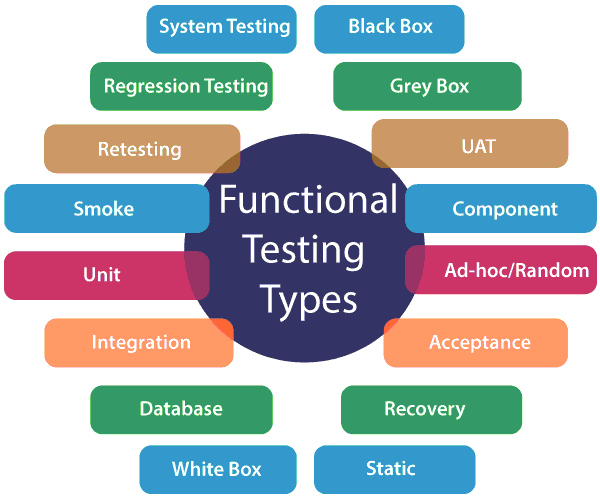
Functional Testing javatpoint
Cap Definition Of Test System Cap believes a laboratory developed. Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Test complexities what is “test complexity”? Cap believes a laboratory developed. Meet required standards from clia, fda and. Clinical laboratory test systems are assigned a moderate or high complexity. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Maintain accuracy of test results and ensure accurate patient diagnosis; Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap laboratory accreditation helps laboratories: Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or.
From helpfulprofessor.com
20 Standardized Tests Pros And Cons (2024) Cap Definition Of Test System Maintain accuracy of test results and ensure accurate patient diagnosis; Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Within its explanation of calibration verification, clia uses reporting range when referring. Cap Definition Of Test System.
From www.simform.com
How to Build a Test Automation Strategy? Cap Definition Of Test System Test complexities what is “test complexity”? Maintain accuracy of test results and ensure accurate patient diagnosis; Clinical laboratory test systems are assigned a moderate or high complexity. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Within its explanation of calibration verification, clia uses reporting range when referring. Cap Definition Of Test System.
From blog.autify.com
What is Unit Testing? Autify Blog Cap Definition Of Test System Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Cap believes a laboratory developed. Clinical laboratory test systems are assigned a moderate or high complexity. Test complexities what is “test complexity”? Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Maintain accuracy. Cap Definition Of Test System.
From www.youtube.com
CAP Theorem System design YouTube Cap Definition Of Test System Clinical laboratory test systems are assigned a moderate or high complexity. Cap laboratory accreditation helps laboratories: Maintain accuracy of test results and ensure accurate patient diagnosis; Meet required standards from clia, fda and. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Within its explanation of calibration verification, clia. Cap Definition Of Test System.
From katalon.com
What is Software Testing? Definition, Importance, and Types Cap Definition Of Test System Clinical laboratory test systems are assigned a moderate or high complexity. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap believes a laboratory developed. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Within. Cap Definition Of Test System.
From www.gremlin.com
Reliability testing Definition, history, methods, and examples Cap Definition Of Test System Cap believes a laboratory developed. Test complexities what is “test complexity”? Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Cap laboratory accreditation helps laboratories: Meet required standards from clia, fda and. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap. Cap Definition Of Test System.
From www.plastikmedia.co.uk
Case Study Vaccine Vials Testing PlastikMedia News Cap Definition Of Test System Meet required standards from clia, fda and. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Maintain accuracy of test results and ensure accurate patient. Cap Definition Of Test System.
From www.youtube.com
Standard test signals in Control System YouTube Cap Definition Of Test System Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Maintain accuracy of test. Cap Definition Of Test System.
From learning-notes.kovacevic.dev
CAP Theorem Learning notes Cap Definition Of Test System Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Test complexities what is “test complexity”? Meet required standards from clia, fda and. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a. Cap Definition Of Test System.
From www.javatpoint.com
Functional Testing javatpoint Cap Definition Of Test System Cap believes a laboratory developed. Clinical laboratory test systems are assigned a moderate or high complexity. Maintain accuracy of test results and ensure accurate patient diagnosis; Test complexities what is “test complexity”? Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia,. Cap Definition Of Test System.
From aplikacebinarnimimoznostmi.logdown.com
Co Je CapAndTrade Systém A Jak To Funguje « Top 3 aplikací s Cap Definition Of Test System Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Meet required standards from clia, fda and. Within its explanation of calibration verification, clia uses reporting range when referring to the span of test. Cap Definition Of Test System.
From bootcamp.uxdesign.cc
My relationship with UI/UX Week 2 by Daniel Crispa Bootcamp Cap Definition Of Test System Clinical laboratory test systems are assigned a moderate or high complexity. Cap laboratory accreditation helps laboratories: Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Test. Cap Definition Of Test System.
From www.deviqa.com
Integration Testing Overview on How to Perform, its Types, and Tools Cap Definition Of Test System Maintain accuracy of test results and ensure accurate patient diagnosis; Test complexities what is “test complexity”? Cap believes a laboratory developed. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an. Cap Definition Of Test System.
From katalon.com
Detailed Guide to Creating a Test Plan Test Plan vs. Test Strategy Cap Definition Of Test System Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Maintain accuracy of test results and ensure accurate patient diagnosis; Test complexities what is “test complexity”? Cap laboratory accreditation helps laboratories: Meet required standards. Cap Definition Of Test System.
From ipsplumbingproducts.com
Test Caps IPS Corporation Plumbing Cap Definition Of Test System Cap believes a laboratory developed. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Clinical laboratory test systems are assigned a moderate or high complexity. Test complexities what is “test complexity”? Cap requires that all high complexity testing personnel have earned at least a minimum of an associate. Cap Definition Of Test System.
From www.simform.com
What is Integration testing? Definition, Tools, and Examples Cap Definition Of Test System Cap laboratory accreditation helps laboratories: Test complexities what is “test complexity”? Clinical laboratory test systems are assigned a moderate or high complexity. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap believes. Cap Definition Of Test System.
From www.learningspiral.co.in
What are Examinations and Examination Systems? Learning Spiral Cap Definition Of Test System Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish. Cap Definition Of Test System.
From juejin.cn
什么是测试环境以及与测试环境相关的主要功能和要素{ 掘金 Cap Definition Of Test System Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Clinical laboratory test systems are assigned a moderate or high complexity. Meet required standards from clia, fda and. Cap believes a laboratory developed. Maintain accuracy of test results and ensure accurate patient diagnosis;. Cap Definition Of Test System.
From www.studocu.com
Biology 5' cap Definition An altered nucleotide that is present at Cap Definition Of Test System Meet required standards from clia, fda and. Cap laboratory accreditation helps laboratories: Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clinical laboratory test systems are assigned. Cap Definition Of Test System.
From www.guru99.com
TEST PLAN in Software Testing (Example) Cap Definition Of Test System Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Test complexities what is “test complexity”? Clinical laboratory test systems are assigned a moderate or high complexity. Maintain accuracy of test results and ensure accurate patient diagnosis; Within its explanation of calibration verification, clia uses reporting range when referring. Cap Definition Of Test System.
From www.slideshare.net
Software testing definition Cap Definition Of Test System Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap believes a laboratory developed. Test complexities what is “test complexity”? Meet required standards from clia, fda and. Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the. Cap Definition Of Test System.
From sampletestcases.com
Use Case Testing Definition, Benefits & Examples Cap Definition Of Test System Maintain accuracy of test results and ensure accurate patient diagnosis; Clinical laboratory test systems are assigned a moderate or high complexity. Cap laboratory accreditation helps laboratories: Cap believes a laboratory developed. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Test complexities what is “test complexity”? Within its explanation of calibration verification, clia uses. Cap Definition Of Test System.
From www.slideshare.net
Testcase definition Cap Definition Of Test System Test complexities what is “test complexity”? Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Clia defines a ‘test system’ as instructions and all the instrumentation,. Cap Definition Of Test System.
From highload.today
User Acceptance Testing (UAT) приймальне тестування та його цілі Cap Definition Of Test System Clinical laboratory test systems are assigned a moderate or high complexity. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap laboratory accreditation helps laboratories: Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Components. Cap Definition Of Test System.
From testsigma.com
Levels of Testing A Complete Approach to Quality Assurance Cap Definition Of Test System Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Meet required standards from clia, fda and. Clinical laboratory test systems are assigned a moderate or high complexity. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory. Cap Definition Of Test System.
From www.analogictips.com
How do MIL, SIL, PIL and HIL simulation and testing relate to MBSE? Cap Definition Of Test System Cap laboratory accreditation helps laboratories: Cap believes a laboratory developed. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Within its explanation of calibration verification, clia. Cap Definition Of Test System.
From www.long-intl.com
Commissioning in Construction Project Startup & Procedures Cap Definition Of Test System Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap believes a laboratory developed. Maintain accuracy of test results and ensure accurate patient diagnosis; Laboratories are required to perform analytical validation or verification. Cap Definition Of Test System.
From themumpreneurshow.com
What Is Test Marketing In Business? The Mumpreneur Show Cap Definition Of Test System Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Meet required standards from clia, fda and. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Test complexities what is “test complexity”? Laboratories are required to perform. Cap Definition Of Test System.
From www.simform.com
What is Integration testing? Definition, Tools, and Examples Cap Definition Of Test System Test complexities what is “test complexity”? Cap believes a laboratory developed. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Maintain accuracy of test results and ensure accurate patient diagnosis; Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Clinical. Cap Definition Of Test System.
From codenboxautomationlab.com
Unit Test What is, Advantages & How works? CodenBox AutomationLab Cap Definition Of Test System Within its explanation of calibration verification, clia uses reporting range when referring to the span of test result values over which the lab can establish or verify. Maintain accuracy of test results and ensure accurate patient diagnosis; Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap believes. Cap Definition Of Test System.
From u-tor.com
System Testing Vs. Integration Testing 6 Key Dissimilarities UTOR Cap Definition Of Test System Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap laboratory accreditation helps laboratories: Meet required standards from clia, fda and. Maintain accuracy of test. Cap Definition Of Test System.
From www.alliedcomponents.com
How to Test a Capacitor Using a Multimeter, an Ohm Meter, and a Volt Meter? Cap Definition Of Test System Meet required standards from clia, fda and. Test complexities what is “test complexity”? Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap believes a. Cap Definition Of Test System.
From www.guru99.com
Qu'estce que l'analyse de test (base de test) dans les tests de Cap Definition Of Test System Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Clinical laboratory test systems are assigned a moderate or high complexity. Meet required standards from clia, fda. Cap Definition Of Test System.
From www.toolsqa.com
What is System Testing? Its Objectives, Test Basics and Test Objects Cap Definition Of Test System Meet required standards from clia, fda and. Components of a laboratory test (ldt) and ldt labeling considerations, and outline our key concerns. Clia defines a ‘test system’ as instructions and all the instrumentation, equipment, reagents, and supplies needed to perform an assay or. Cap laboratory accreditation helps laboratories: Maintain accuracy of test results and ensure accurate patient diagnosis; Cap requires. Cap Definition Of Test System.
From www.gocd.org
Five Considerations for Continuous Delivery of Microservices GoCD Blog Cap Definition Of Test System Cap believes a laboratory developed. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Maintain accuracy of test results and ensure accurate patient diagnosis; Test complexities. Cap Definition Of Test System.