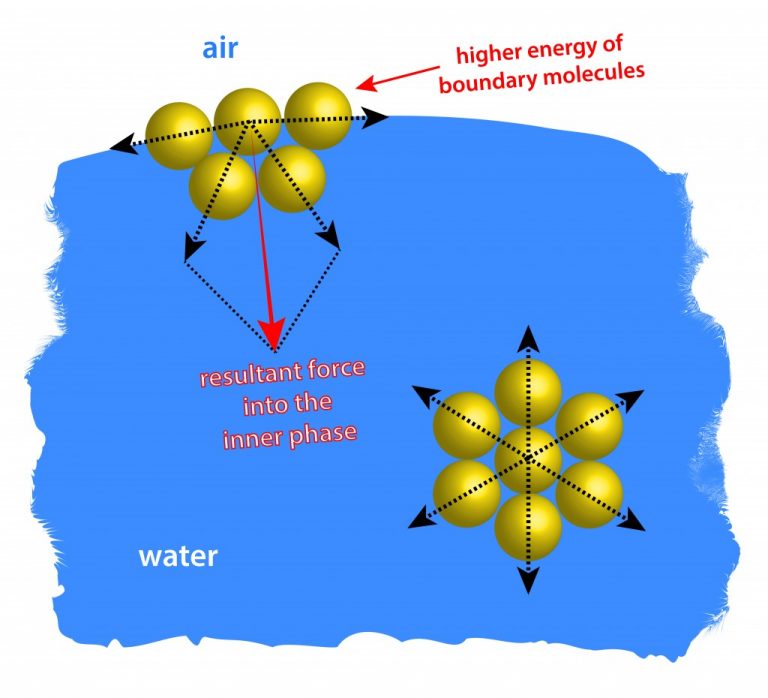
Explain Surface Tension On The Basis Of Molecular Theory . Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. Since these intermolecular forces vary depending on the nature of the. In the theory of surface phenomena [1, 6]. The surface tension of a liquid results from an imbalance of. It is defined as the force. Molecular theory of surface tension: Ps is the free surface of the liquid and. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. Let pqrs = surface film of liquid in a container containing liquid. The molecules at the surface do not have other like. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the.
from www.scienceabc.com
The surface tension of a liquid results from an imbalance of. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The molecules at the surface do not have other like. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Ps is the free surface of the liquid and. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. Molecular theory of surface tension: It is defined as the force. In the theory of surface phenomena [1, 6]. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.
Surface Tension Definition, Explanation, Examples And Significance
Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. Let pqrs = surface film of liquid in a container containing liquid. The molecules at the surface do not have other like. Molecular theory of surface tension: In the theory of surface phenomena [1, 6]. The surface tension of a liquid results from an imbalance of. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Since these intermolecular forces vary depending on the nature of the. Ps is the free surface of the liquid and. It is defined as the force. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per.
From www.toppr.com
Chapter 6. Surface Tension Q. 9. Explain the phenomenon of surface Explain Surface Tension On The Basis Of Molecular Theory The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. The surface tension of a liquid results from an imbalance of. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Explain Surface Tension On The Basis Of Molecular Theory.
From mackenzie-has-adkins.blogspot.com
Describe What Surface Tension Is Using Theory Explain Surface Tension On The Basis Of Molecular Theory The surface tension of a liquid results from an imbalance of. Molecular theory of surface tension: Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the. Explain Surface Tension On The Basis Of Molecular Theory.
From askfilo.com
38. Explain surface tension on the basis of molecular theory. Filo Explain Surface Tension On The Basis Of Molecular Theory Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. In the theory of surface phenomena [1, 6]. Molecular theory of surface tension: Let pqrs =. Explain Surface Tension On The Basis Of Molecular Theory.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Explain Surface Tension On The Basis Of Molecular Theory Since these intermolecular forces vary depending on the nature of the. The surface tension of a liquid results from an imbalance of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It is defined as the force. The molecules at the surface do not have other like. The cohesive. Explain Surface Tension On The Basis Of Molecular Theory.
From byjus.com
What is surface tension? Explain Surface Tension On The Basis Of Molecular Theory Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The molecules at the surface do not have other like. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Let pqrs =. Explain Surface Tension On The Basis Of Molecular Theory.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Explain Surface Tension On The Basis Of Molecular Theory In the theory of surface phenomena [1, 6]. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Molecular theory of surface. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Surface Tension on The Basis of Molecular TheoryMechanical Properties Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Let pqrs = surface film of liquid in a container containing liquid. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface do not have other like. It is defined. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Surface tension on the basis of a molecular theory YouTube Explain Surface Tension On The Basis Of Molecular Theory Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The surface tension of a liquid results from an imbalance of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is measured as the energy required to increase. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Explanation of surface tension on the basis of molecular theory YouTube Explain Surface Tension On The Basis Of Molecular Theory The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of. Explain Surface Tension On The Basis Of Molecular Theory.
From www.slideserve.com
PPT Physics 221, March 15 PowerPoint Presentation ID1597550 Explain Surface Tension On The Basis Of Molecular Theory Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Ps is the free surface of the liquid and. Let pqrs = surface film of liquid in a container containing liquid. Molecular theory of. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
2.4.1 Molecular Theory of Surface Tension YouTube Explain Surface Tension On The Basis Of Molecular Theory It is defined as the force. Let pqrs = surface film of liquid in a container containing liquid. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The surface tension of a liquid results from an imbalance of. The molecules at. Explain Surface Tension On The Basis Of Molecular Theory.
From www.toppr.com
Explain the formation of concave and convex surface of liquid on the Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The molecules at the surface do not have other like. The cohesive forces between liquid molecules are responsible for the phenomenon. Explain Surface Tension On The Basis Of Molecular Theory.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Explain Surface Tension On The Basis Of Molecular Theory The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. In the theory of surface phenomena [1, 6]. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Ps is the free surface of. Explain Surface Tension On The Basis Of Molecular Theory.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Explain Surface Tension On The Basis Of Molecular Theory The surface tension of a liquid results from an imbalance of. Let pqrs = surface film of liquid in a container containing liquid. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Molecular theory of surface tension: In the theory of surface phenomena [1, 6]. Surface tension is. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Surface Tension on the basis of Molecular Theory 2020 Hindi YouTube Explain Surface Tension On The Basis Of Molecular Theory In the theory of surface phenomena [1, 6]. The surface tension of a liquid results from an imbalance of. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Since these intermolecular forces vary depending on the nature of the. Ps is. Explain Surface Tension On The Basis Of Molecular Theory.
From www.numerade.com
SOLVED Explain surface tension on the basis of molecular theory. Explain Surface Tension On The Basis Of Molecular Theory The molecules at the surface do not have other like. The surface tension of a liquid results from an imbalance of. Let pqrs = surface film of liquid in a container containing liquid. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on. Explain Surface Tension On The Basis Of Molecular Theory.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension Explain Surface Tension On The Basis Of Molecular Theory Since these intermolecular forces vary depending on the nature of the. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between. Explain Surface Tension On The Basis Of Molecular Theory.
From byjus.com
Explain surface tension on the basis of molecular theory. Explain Surface Tension On The Basis Of Molecular Theory The molecules at the surface do not have other like. Since these intermolecular forces vary depending on the nature of the. In the theory of surface phenomena [1, 6]. Ps is the free surface of the liquid and. The surface tension of a liquid results from an imbalance of. The surface tension is the physical property experienced by a liquid. Explain Surface Tension On The Basis Of Molecular Theory.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Explain Surface Tension On The Basis Of Molecular Theory The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. It is defined as the force. In the theory of surface phenomena [1, 6]. Surface tension is measured as the energy required to increase the surface. Explain Surface Tension On The Basis Of Molecular Theory.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Ps is the free surface of the liquid and. The cohesive forces. Explain Surface Tension On The Basis Of Molecular Theory.
From www.pinterest.com
surface tension Surface tension, Chemistry lessons, Surface Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Molecular theory of surface tension: The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. The cohesive forces between liquid molecules are responsible for the. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Surface tension on the basis of molecular theory YouTube Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Let pqrs = surface film of liquid in a container containing liquid. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the. Explain Surface Tension On The Basis Of Molecular Theory.
From owlcation.com
Primary and Secondary Bonds Owlcation Explain Surface Tension On The Basis Of Molecular Theory It is defined as the force. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. Surface tension is the energy required to increase the surface. Explain Surface Tension On The Basis Of Molecular Theory.
From www.doubtnut.com
[Tamil] Explain surface tension on the basis of molecular theory. Explain Surface Tension On The Basis Of Molecular Theory Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Ps is the free surface of the liquid and. In the. Explain Surface Tension On The Basis Of Molecular Theory.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Explain Surface Tension On The Basis Of Molecular Theory Let pqrs = surface film of liquid in a container containing liquid. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules at the surface do not. Explain Surface Tension On The Basis Of Molecular Theory.
From worksheetschoolfaust.z13.web.core.windows.net
How To Explain Surface Tension To Kids Explain Surface Tension On The Basis Of Molecular Theory Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. Let pqrs = surface film of liquid in a container containing liquid. Molecular theory of surface tension: Surface tension is. Explain Surface Tension On The Basis Of Molecular Theory.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Explain Surface Tension On The Basis Of Molecular Theory The molecules at the surface do not have other like. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. It is defined as the force. Ps is the free surface of the liquid and. The surface tension of a liquid results from an imbalance of. Since these intermolecular. Explain Surface Tension On The Basis Of Molecular Theory.
From www.meritnation.com
Solve this 5 4 Explanation of surface Tension on the Basis of Explain Surface Tension On The Basis Of Molecular Theory Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Let pqrs = surface film of liquid in a container containing liquid. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension (st) is defined as the. Explain Surface Tension On The Basis Of Molecular Theory.
From aznswerzonelinclarifying.z21.web.core.windows.net
How To Explain Surface Tension Explain Surface Tension On The Basis Of Molecular Theory It is defined as the force. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Molecular theory of surface tension: Surface tension (st) is defined as. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
surface Tension on the basis of molecular theory part 2 lecture 3 Explain Surface Tension On The Basis Of Molecular Theory The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Since these intermolecular forces vary depending on the nature of the. In the theory of surface phenomena [1, 6]. The surface tension is the. Explain Surface Tension On The Basis Of Molecular Theory.
From aznswerzonelinclarifying.z21.web.core.windows.net
How To Explain Surface Tension Explain Surface Tension On The Basis Of Molecular Theory Ps is the free surface of the liquid and. Since these intermolecular forces vary depending on the nature of the. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension of a liquid results from an imbalance of. Let pqrs = surface film of liquid in. Explain Surface Tension On The Basis Of Molecular Theory.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts Explain Surface Tension On The Basis Of Molecular Theory The molecules at the surface do not have other like. The surface tension of a liquid results from an imbalance of. Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the. Explain Surface Tension On The Basis Of Molecular Theory.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube Explain Surface Tension On The Basis Of Molecular Theory The molecules at the surface do not have other like. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension (st) is defined as the. Explain Surface Tension On The Basis Of Molecular Theory.
From aznswerzonelinclarifying.z21.web.core.windows.net
How To Explain Surface Tension Explain Surface Tension On The Basis Of Molecular Theory In the theory of surface phenomena [1, 6]. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the. Since these intermolecular forces vary depending on the nature of the. The cohesive forces between liquid molecules are responsible for the phenomenon known as. Explain Surface Tension On The Basis Of Molecular Theory.
From www.toppr.com
Chapter 6. Surface Tension Q. 9. Explain the phenomenon of surface Explain Surface Tension On The Basis Of Molecular Theory Surface tension (st) is defined as the work required tо create an interface unit (j/m2) or the force per. It is defined as the force. The surface tension is the physical property experienced by a liquid due to the free or unbonded molecules along the boundaries of the liquid container. Surface tension is measured as the energy required to increase. Explain Surface Tension On The Basis Of Molecular Theory.