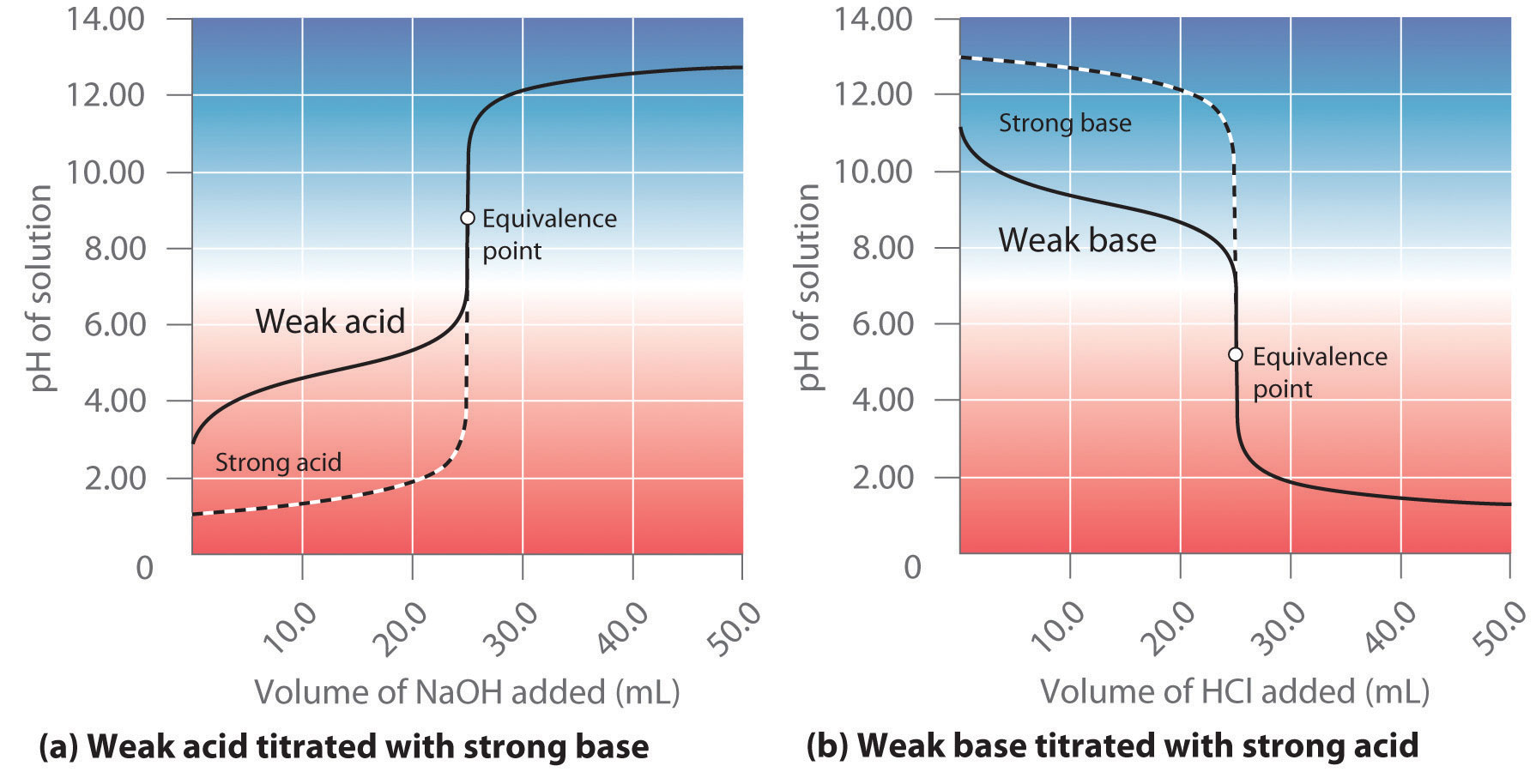
Titration Graph Trends . Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The figure below shows two different examples of a strong. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph meter can be used and a ph curve plotted; For example, titrating sodium hydroxide against ethanoic. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. A graph showing the change in ph during a titration is called a titration curve.
from chem.libretexts.org
Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. During a titration a ph meter can be used and a ph curve plotted; Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. The figure below shows two different examples of a strong. For example, titrating sodium hydroxide against ethanoic. A ph curve is a graph showing how the ph of a solution changes as the acid (or. A graph showing the change in ph during a titration is called a titration curve.
15.6 AcidBase Titration Curves Chemistry LibreTexts
Titration Graph Trends For example, titrating sodium hydroxide against ethanoic. For example, titrating sodium hydroxide against ethanoic. The equivalence point of a titration. A ph curve is a graph showing how the ph of a solution changes as the acid (or. The figure below shows two different examples of a strong. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A graph showing the change in ph during a titration is called a titration curve. During a titration a ph meter can be used and a ph curve plotted;
From maxinecieran.blogspot.com
Redox titration graph MaxineCieran Titration Graph Trends Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. During a titration a ph meter can be used and a ph curve plotted; For example, titrating sodium hydroxide against ethanoic. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during. Titration Graph Trends.
From saylordotorg.github.io
AcidBase Titrations Titration Graph Trends For example, titrating sodium hydroxide against ethanoic. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The equivalence point of a titration. A graph showing the change in ph during a titration is called a titration curve. Titration or ph curves are obtained by reacting known concentrations of acid. Titration Graph Trends.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Graph Trends A ph curve is a graph showing how the ph of a solution changes as the acid (or. The figure below shows two different examples of a strong. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A titration curve is a graphical representation of. Titration Graph Trends.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Graph Trends The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. For example, titrating sodium hydroxide against ethanoic. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A ph curve is a graph showing how the ph of. Titration Graph Trends.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. For example, titrating sodium hydroxide against ethanoic. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. During a titration a ph meter can be used. Titration Graph Trends.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Titration Graph Trends The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. A ph curve is a graph showing how the ph of a solution changes as the acid (or. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution.. Titration Graph Trends.
From www.researchgate.net
a is the potentiometric titration graph of free Fe 3+ . In this graph Titration Graph Trends The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. Titration is a technique used in neutralisation reactions. Titration Graph Trends.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. For example, titrating sodium hydroxide against ethanoic. The figure. Titration Graph Trends.
From www.pearson.com
The graph shows the titration curves for two monopro Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. For example, titrating sodium hydroxide against ethanoic. The equivalence point of a titration. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. The figure below shows two different examples of a strong. During a. Titration Graph Trends.
From www.chegg.com
Solved Look at the titration graphs below. Determine which Titration Graph Trends The equivalence point of a titration. The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. For example, titrating sodium hydroxide against ethanoic. A titration curve is a graphical representation of the ph of a solution during a titration. Titration. Titration Graph Trends.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Titration Graph Trends Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. During a titration a ph meter can be used and a ph curve plotted; For example,. Titration Graph Trends.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. The equivalence point of a titration. The figure below shows two different examples of a strong.. Titration Graph Trends.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Titration Graph Trends A ph curve is a graph showing how the ph of a solution changes as the acid (or. A titration curve is a graphical representation of the ph of a solution during a titration. For example, titrating sodium hydroxide against ethanoic. A graph showing the change in ph during a titration is called a titration curve. The figure below shows. Titration Graph Trends.
From www.chegg.com
Solved The graphs labeled (a) and (b) show the titration Titration Graph Trends During a titration a ph meter can be used and a ph curve plotted; Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. A graph showing the. Titration Graph Trends.
From www.pearson.com
The graphs labeled (a) and (b) show the titration curves for two Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. A ph curve is a graph showing how the ph of a solution changes as the acid (or. For example, titrating sodium hydroxide against ethanoic. The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known concentrations. Titration Graph Trends.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. A graph showing the change in ph during a titration is called a titration. Titration Graph Trends.
From www.xylemanalytics.com
How to get correct and reproducible results in titration Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A ph curve is a graph showing how the ph of a solution changes as the acid (or. During a titration a ph meter can be used and a ph curve plotted; Titration is a technique. Titration Graph Trends.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Graph Trends During a titration a ph meter can be used and a ph curve plotted; Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A graph showing the change in ph during a titration is called a titration curve. A titration curve is a graph that relates the change in. Titration Graph Trends.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. During a titration a ph meter can be used and a ph curve plotted; The figure below shows two different examples of a strong. For example, titrating sodium hydroxide against ethanoic. A titration curve is a graph that relates the change in ph of an. Titration Graph Trends.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. A ph curve is a graph showing how the ph of a solution changes. Titration Graph Trends.
From university.pressbooks.pub
15.7 AcidBase Titrations Chemistry Fundamentals Titration Graph Trends For example, titrating sodium hydroxide against ethanoic. The equivalence point of a titration. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graph that relates the change in. Titration Graph Trends.
From chemistnotes.com
Conductometric titration, easy principle, curves, 3 advantages Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. During a titration a ph meter can be used and a ph curve plotted; The equivalence point of a titration. For example, titrating sodium hydroxide against ethanoic. A titration curve is a graphical representation of the ph of a solution during a titration. Titration or. Titration Graph Trends.
From chem.libretexts.org
9.5 Precipitation Titrations Chemistry LibreTexts Titration Graph Trends A ph curve is a graph showing how the ph of a solution changes as the acid (or. The figure below shows two different examples of a strong. During a titration a ph meter can be used and a ph curve plotted; For example, titrating sodium hydroxide against ethanoic. Titration or ph curves are obtained by reacting known concentrations of. Titration Graph Trends.
From www.jove.com
AcidBase Titration Curves JoVE Titration Graph Trends A ph curve is a graph showing how the ph of a solution changes as the acid (or. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. During a. Titration Graph Trends.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Graph Trends During a titration a ph meter can be used and a ph curve plotted; For example, titrating sodium hydroxide against ethanoic. The equivalence point of a titration. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. Titration is a technique used in neutralisation reactions between acids and alkalis to. Titration Graph Trends.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Graph Trends A graph showing the change in ph during a titration is called a titration curve. The equivalence point of a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known. Titration Graph Trends.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Graph Trends The equivalence point of a titration. The figure below shows two different examples of a strong. A ph curve is a graph showing how the ph of a solution changes as the acid (or. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A graph showing the change in. Titration Graph Trends.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Graph Trends A titration curve is a graphical representation of the ph of a solution during a titration. A graph showing the change in ph during a titration is called a titration curve. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. The equivalence point of a. Titration Graph Trends.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry Titration Graph Trends The figure below shows two different examples of a strong. A graph showing the change in ph during a titration is called a titration curve. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. A titration curve is a graphical representation of the ph of a solution during a. Titration Graph Trends.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Graph Trends During a titration a ph meter can be used and a ph curve plotted; The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. The equivalence point of a titration. For example, titrating sodium hydroxide against ethanoic. A titration curve. Titration Graph Trends.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Graph Trends Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. For example, titrating sodium hydroxide against ethanoic. A graph showing the change in ph during a titration is called a titration curve. The figure below shows two different examples of a strong. A titration curve is a graph that relates. Titration Graph Trends.
From www.youtube.com
Conductometric titration of strong acid and weak base (strong acid vs Titration Graph Trends The figure below shows two different examples of a strong. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. During a titration a ph meter can be used and a ph curve plotted; A graph showing the change in ph during a titration is called a titration curve. A. Titration Graph Trends.
From schoolbag.info
Figure 10.11. Strong Acid and Weak Base Titration Curve A strong acid Titration Graph Trends Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph during the. During a titration a ph meter can be used and a ph curve plotted; A graph showing the change in. Titration Graph Trends.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Titration Graph Trends A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A graph showing the change in ph during a titration is called a titration curve. A ph curve is a graph showing how the ph of a solution changes as the acid (or. A titration curve. Titration Graph Trends.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Graph Trends A ph curve is a graph showing how the ph of a solution changes as the acid (or. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Titration or ph curves are obtained by reacting known concentrations of acid with alkalis and measuring the ph. Titration Graph Trends.