Chlorine Electronegativity Value . It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. In this the coefficient of electronegativity is defined as: The electronegativity of chlorine is: It can also be used to predict if the resulting molecule will be polar. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. The force of electrostatic attraction that you experience an. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The suggested values are all taken from. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is among the highly reactive non.
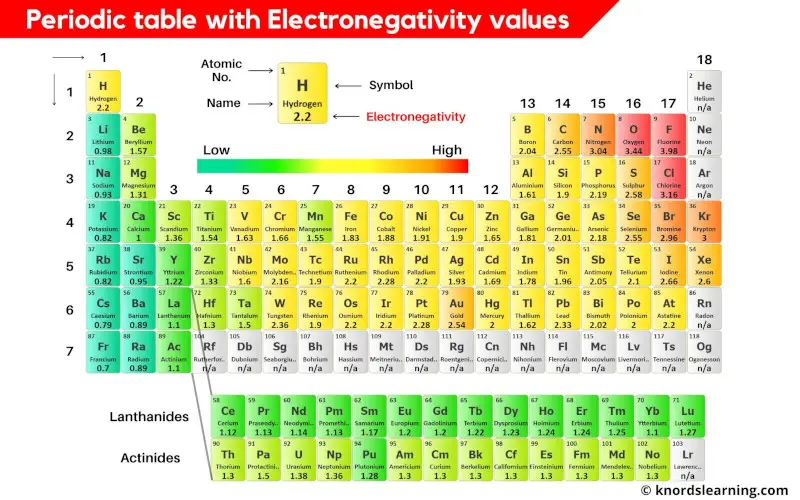
from knordslearning.com
3.16 is the electronegativity value of chlorine (cl). In this the coefficient of electronegativity is defined as: It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. The electronegativity of chlorine is: Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The suggested values are all taken from. The force of electrostatic attraction that you experience an. 103 rows electronegativity is not a uniquely defined property and may depend on the definition.
Periodic Table with Electronegativity Values (Labeled Image)
Chlorine Electronegativity Value The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. It is among the highly reactive non. In this the coefficient of electronegativity is defined as: It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. The force of electrostatic attraction that you experience an. The electronegativity of chlorine is: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar. The suggested values are all taken from. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence.
From klavljohb.blob.core.windows.net
Potassium Chlorine Electronegativity Difference at Alvin Hughes blog Chlorine Electronegativity Value It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). It can also be used to predict if the resulting molecule will be polar. It is among the highly reactive non. It is caused by the attractive electrostatic force between the positively charged nucleus and. Chlorine Electronegativity Value.
From mungfali.com
Electronegativity Chart And Lewis Structures Chlorine Electronegativity Value The force of electrostatic attraction that you experience an. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. The suggested values are all taken from. Electronegativity is the tendency of an atom. Chlorine Electronegativity Value.
From www.sliderbase.com
Periodic Table of Electronegativities Chlorine Electronegativity Value In this the coefficient of electronegativity is defined as: It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. It is among the highly reactive non. 103 rows electronegativity is not a uniquely defined. Chlorine Electronegativity Value.
From socratic.org
Periodic Trends in Electronegativity Chemistry Socratic Chlorine Electronegativity Value It can also be used to predict if the resulting molecule will be polar. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. In this the. Chlorine Electronegativity Value.
From www.numerade.com
SOLVED Is CCla a polar or nonpolar molecule and why? Electronegativity Chlorine Electronegativity Value In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. It can also be used to predict if the resulting molecule will be polar. 103 rows electronegativity is not a uniquely defined property. Chlorine Electronegativity Value.
From periodictableguide.com
Periodic table with Electronegativity Values (Labeled Image) Chlorine Electronegativity Value 3.16 is the electronegativity value of chlorine (cl). Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. It belongs to the 7th group and 2nd period on the periodic table, known as the. Chlorine Electronegativity Value.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Chlorine Electronegativity Value 3.16 is the electronegativity value of chlorine (cl). The force of electrostatic attraction that you experience an. It is among the highly reactive non. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. In general, an atom’s. Chlorine Electronegativity Value.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine Electronegativity Value In this the coefficient of electronegativity is defined as: The force of electrostatic attraction that you experience an. 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a. Chlorine Electronegativity Value.
From klavljohb.blob.core.windows.net
Potassium Chlorine Electronegativity Difference at Alvin Hughes blog Chlorine Electronegativity Value It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The electronegativity of chlorine is: It is among the highly reactive non. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The force of electrostatic attraction that you experience an. 119 rows electronegativity is used to predict. Chlorine Electronegativity Value.
From general.chemistrysteps.com
CH3Cl Polar or Nonpolar Chemistry Steps Chlorine Electronegativity Value It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In this the coefficient of electronegativity is defined as: In general, an atom’s electronegativity is affected by both its atomic number and the distance at which. Chlorine Electronegativity Value.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Electronegativity Value The force of electrostatic attraction that you experience an. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. In this the coefficient of electronegativity is defined as: 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The electronegativity of chlorine is: The suggested values are all. Chlorine Electronegativity Value.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Chlorine Electronegativity Value It is among the highly reactive non. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its. Chlorine Electronegativity Value.
From wisc.pb.unizin.org
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104 Chlorine Electronegativity Value 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In this the coefficient of electronegativity is defined as: It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It is among the highly reactive non. In general, an atom’s electronegativity is affected by both its. Chlorine Electronegativity Value.
From ar.inspiredpencil.com
Electronegativity Values Chlorine Electronegativity Value The electronegativity of chlorine is: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. In this the coefficient. Chlorine Electronegativity Value.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Chlorine Electronegativity Value The suggested values are all taken from. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It can also be used to predict if the resulting molecule will be polar. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. The first scale. Chlorine Electronegativity Value.
From mavink.com
Element Electronegativity Chart Chlorine Electronegativity Value The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. 3.16 is the electronegativity value of chlorine (cl). The suggested values are all taken from. In this the coefficient of electronegativity is defined as: It is caused by the attractive electrostatic force between the. Chlorine Electronegativity Value.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Electronegativity Value 3.16 is the electronegativity value of chlorine (cl). The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. The suggested values are all taken from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In general,. Chlorine Electronegativity Value.
From knordslearning.com
Periodic Table with Electronegativity Values (Labeled Image) Chlorine Electronegativity Value It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. The force of electrostatic attraction that you experience an. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It. Chlorine Electronegativity Value.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Electronegativity Value The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. Electronegativity is the tendency of an atom to attract a pair of electrons in a. Chlorine Electronegativity Value.
From studylib.net
electronegativity and polar covalent bonds Chlorine Electronegativity Value The suggested values are all taken from. In this the coefficient of electronegativity is defined as: In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from. Chlorine Electronegativity Value.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Chlorine Electronegativity Value In this the coefficient of electronegativity is defined as: It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. In general, an atom’s electronegativity is affected by both its atomic number and the distance at. Chlorine Electronegativity Value.
From wisc.pb.unizin.org
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104 Chlorine Electronegativity Value It can also be used to predict if the resulting molecule will be polar. The electronegativity of chlorine is: 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is among the highly reactive non. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of. Chlorine Electronegativity Value.
From www.sampletemplates.com
FREE 13+ Sample Electronegativity Chart Templates in PDF MS Word Excel Chlorine Electronegativity Value It can also be used to predict if the resulting molecule will be polar. The electronegativity of chlorine is: It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). In this the coefficient of electronegativity is defined as: It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.. Chlorine Electronegativity Value.
From www.pinterest.com
Electronegativity Definition and Trend Chlorine Electronegativity Value 3.16 is the electronegativity value of chlorine (cl). It can also be used to predict if the resulting molecule will be polar. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is among the highly reactive non. In general, an atom’s electronegativity is affected by both its atomic number and the distance at. Chlorine Electronegativity Value.
From cartoondealer.com
Chlorine Chemical Element With 17 Atomic Number, Atomic Mass And Chlorine Electronegativity Value The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The electronegativity of chlorine is: Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. The force of electrostatic attraction that you experience an. The first scale of electronegativity was developed. Chlorine Electronegativity Value.
From klavljohb.blob.core.windows.net
Potassium Chlorine Electronegativity Difference at Alvin Hughes blog Chlorine Electronegativity Value The force of electrostatic attraction that you experience an. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. The suggested values are all taken from. In this the coefficient of electronegativity is defined as: It can also be used to predict if the resulting molecule will be polar. The first. Chlorine Electronegativity Value.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Electronegativity Value In this the coefficient of electronegativity is defined as: The force of electrostatic attraction that you experience an. It can also be used to predict if the resulting molecule will be polar. It is among the highly reactive non. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. Electronegativity is the. Chlorine Electronegativity Value.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Chlorine Electronegativity Value 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The force of electrostatic attraction that you experience an. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. The electronegativity of chlorine is: The first scale of electronegativity was developed by linus pauling and on his. Chlorine Electronegativity Value.
From www.numerade.com
SOLVED The bond between sulfur (electronegativity value 2.5) and Chlorine Electronegativity Value It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The force of electrostatic attraction that you experience an. The suggested values are all taken from. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is caused by the attractive electrostatic force between the positively charged. Chlorine Electronegativity Value.
From www.doubtnut.com
Calculate the electronegativity value of chlorine on Mulliken's scale Chlorine Electronegativity Value 3.16 is the electronegativity value of chlorine (cl). The force of electrostatic attraction that you experience an. The suggested values are all taken from. It can also be used to predict if the resulting molecule will be polar. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used. Chlorine Electronegativity Value.
From www.youtube.com
Calculate the electronegativity value of chlorine on Mulliken\'s scale Chlorine Electronegativity Value In this the coefficient of electronegativity is defined as: Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which. Chlorine Electronegativity Value.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Electronegativity Value It can also be used to predict if the resulting molecule will be polar. It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. The force of electrostatic attraction that you experience an. The electronegativity of chlorine is:. Chlorine Electronegativity Value.
From mungfali.com
Periodic Table Of Elements Electronegativity Chlorine Electronegativity Value 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It is among the highly reactive non. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The first scale of. Chlorine Electronegativity Value.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Chlorine Electronegativity Value In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence. It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). The suggested values are all taken from. It can also be used to predict if the resulting molecule will be polar. Electronegativity is the tendency of. Chlorine Electronegativity Value.
From www.periodic-table.org
Chlorine Electronegativity Cl Chlorine Electronegativity Value It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). It can also be used to predict if the resulting molecule will be polar. The first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale running from from. The suggested values are all. Chlorine Electronegativity Value.