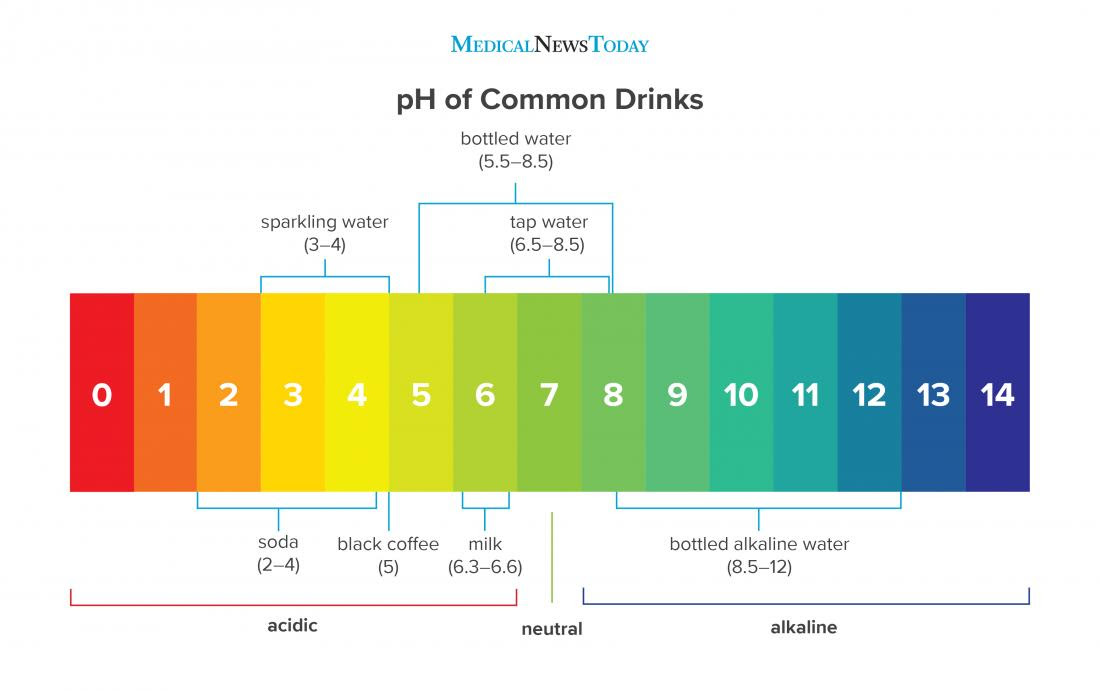
What Does Ph Of Water Stand For . Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The term, widely used in chemistry, biology, and agronomy, translates the. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph is a measure of how acidic/basic water is. Ph of 7 is considered neutral (e.g., pure water). Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The range goes from 0 to 14, with 7 being neutral.
from news.regenerativemedgroup.com
Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph of 7 is considered neutral (e.g., pure water). Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. The range goes from 0 to 14, with 7 being neutral. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic/basic water is. The term, widely used in chemistry, biology, and agronomy, translates the.
The pH of water What to know
What Does Ph Of Water Stand For The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. The range goes from 0 to 14, with 7 being neutral. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the. Ph is a measure of how acidic/basic water is. Ph of 7 is considered neutral (e.g., pure water). Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids.
From www.expii.com
What Is pH? — Definition & Overview Expii What Does Ph Of Water Stand For Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a. What Does Ph Of Water Stand For.
From netsolwater.com
What is pH in water, and what is pH in wastewater? Netsol Water What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic/basic water is. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph. What Does Ph Of Water Stand For.
From www.survivopedia.com
How To Measure Water pH At Home Survivopedia What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The range goes from 0 to 14, with 7 being neutral. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph of 7 is considered neutral (e.g., pure water). Although the ph of pure water. What Does Ph Of Water Stand For.
From naturalbiohealth.com
How Learning the pH Scale Can Create a More Balanced Diet Natural Bio What Does Ph Of Water Stand For Ph is a measure of how acidic/basic water is. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph, quantitative measure of the acidity or basicity of. What Does Ph Of Water Stand For.
From aquaclearws.com
Why Is The pH of Water Important? Aqua Clear Water Systems What Does Ph Of Water Stand For The range goes from 0 to 14, with 7 being neutral. The term, widely used in chemistry, biology, and agronomy, translates the. Ph is a measure of how acidic/basic water is. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph stands for power of hydrogen. the h is capitalized because it is the. What Does Ph Of Water Stand For.
From water.mytyent.com
Alkaline Water pH Levels A Quick Guide TyentUSA Water Ionizer Health What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. The. What Does Ph Of Water Stand For.
From printablelibpathos.z13.web.core.windows.net
How To Calculate Ph On Calculator What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. The range goes from 0 to 14, with 7 being neutral. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a. What Does Ph Of Water Stand For.
From animalia-life.club
Ph Scale Horizontal What Does Ph Of Water Stand For Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph of 7 is considered neutral (e.g., pure water). Ph is a measure of how acidic/basic water is. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. The range goes. What Does Ph Of Water Stand For.
From drowwater.com
Best pH Level for Drinking Water? DrowWater What Does Ph Of Water Stand For The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph is a measure of how acidic/basic water is. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The range goes from 0 to 14, with 7 being neutral. The. What Does Ph Of Water Stand For.
From skfelixer.com
The pH Value Of Purified Water — All You Need To Know SKF Elixer What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. The range goes from. What Does Ph Of Water Stand For.
From www.reefaquarium.com
pH in Marine Aquariums Reef Aquarium What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and. What Does Ph Of Water Stand For.
From www.plumbingsupply.com
Water pH and Your Plumbing What Does Ph Of Water Stand For The term, widely used in chemistry, biology, and agronomy, translates the. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The range goes from 0 to 14, with 7 being neutral. Ph of 7 is considered neutral (e.g., pure water). The ph scale shows the leaves of acidity and alkalinity of water. What Does Ph Of Water Stand For.
From www.careerpower.in
pH Values List for Common Substances What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph, quantitative measure of the acidity or basicity of aqueous or. What Does Ph Of Water Stand For.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Does Ph Of Water Stand For Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. The range goes from 0 to 14, with 7 being neutral. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases.. What Does Ph Of Water Stand For.
From www.australianenvironmentaleducation.com.au
What is pH? pH is the abbreviation of the potential of Hydrogen What Does Ph Of Water Stand For Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph of 7 is considered neutral (e.g., pure water). Phs of less than 7 indicate acidity, whereas a ph. What Does Ph Of Water Stand For.
From www.waterev.com
How to Lower pH In water What Does Ph Of Water Stand For Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. The term, widely used. What Does Ph Of Water Stand For.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Does Ph Of Water Stand For The term, widely used in chemistry, biology, and agronomy, translates the. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because. What Does Ph Of Water Stand For.
From bahamaswater.in
How do you determine what pH level is safe for drinking? What Does Ph Of Water Stand For Ph is a measure of how acidic/basic water is. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. The range goes from 0 to 14, with 7 being neutral. Ph. What Does Ph Of Water Stand For.
From netsolwater.com
What is pH in water? How is pH level measured What Does Ph Of Water Stand For The term, widely used in chemistry, biology, and agronomy, translates the. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or. What Does Ph Of Water Stand For.
From www.slideserve.com
PPT What is pH? PowerPoint Presentation, free download ID5570624 What Does Ph Of Water Stand For Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph is a. What Does Ph Of Water Stand For.
From keywordsuggest.org
Image Gallery hydrochloric acid ph What Does Ph Of Water Stand For Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Phs of less than 7 indicate acidity, whereas a ph of. What Does Ph Of Water Stand For.
From www.bottlingindia.com
What is pH of Water? Everything You Should Know About Water What Does Ph Of Water Stand For The term, widely used in chemistry, biology, and agronomy, translates the. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph of 7 is considered neutral (e.g., pure water). Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Ph,. What Does Ph Of Water Stand For.
From www.worksheetsplanet.com
What is PH Definition What Does Ph Of Water Stand For The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. Ph is a measure of how acidic/basic water is. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph of 7 is considered neutral (e.g., pure water). Ph, quantitative measure of the acidity or basicity of aqueous. What Does Ph Of Water Stand For.
From sciencetrends.com
What Does pH Stand For And Mean? Science Trends What Does Ph Of Water Stand For Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the. The. What Does Ph Of Water Stand For.
From ansa-na.com
The Importance of Water pH to Global Water Quality ANSA North America What Does Ph Of Water Stand For Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph is a measure of how acidic/basic water is. The term, widely used in chemistry, biology, and agronomy, translates. What Does Ph Of Water Stand For.
From drinkprime.in
Best PH Level for drinking water Pani ka ph maan What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph describes how acidic or basic an aqueous solution is, where a. What Does Ph Of Water Stand For.
From www.slideserve.com
PPT Water Chemistry PowerPoint Presentation, free download ID5763752 What Does Ph Of Water Stand For Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The term, widely used in chemistry, biology, and agronomy, translates the.. What Does Ph Of Water Stand For.
From finlaybuckley.z19.web.core.windows.net
Water Ph Color Chart What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The term, widely used in chemistry, biology, and agronomy, translates the. Ph is a measure of how acidic/basic water is. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and. What Does Ph Of Water Stand For.
From lucianobrionesjr.blogspot.com
Luciano A.S. Blog Properties of Water pH video What Does Ph Of Water Stand For Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph of 7 is considered neutral (e.g., pure water). Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. The ph scale shows the leaves of acidity and alkalinity of water and similar liquids. The range. What Does Ph Of Water Stand For.
From watertechadvice.com
pH of Water Everything You Need To Know What Does Ph Of Water Stand For The range goes from 0 to 14, with 7 being neutral. Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Ph is a measure of how acidic/basic water is. The term, widely used in chemistry, biology, and agronomy, translates the. Ph, quantitative measure of the acidity or basicity of aqueous or other. What Does Ph Of Water Stand For.
From www.intec-america.com
Things You Must Know About pH Control and Drinking Water Treatment What Does Ph Of Water Stand For The range goes from 0 to 14, with 7 being neutral. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Ph is a measure of how acidic/basic water is. Although. What Does Ph Of Water Stand For.
From mungfali.com
Ph Chart For Water What Does Ph Of Water Stand For Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. The ph. What Does Ph Of Water Stand For.
From news.regenerativemedgroup.com
The pH of water What to know What Does Ph Of Water Stand For Ph describes how acidic or basic an aqueous solution is, where a ph below 7 is acidic and a ph greater than 7 is basic. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic/basic water is. The range goes from 0 to 14, with 7 being neutral. Ph. What Does Ph Of Water Stand For.
From zanyplantworld.com
PH Water Levels For The Plants Definition, Benefits, And Effects What Does Ph Of Water Stand For Ph of 7 is considered neutral (e.g., pure water). Ph stands for power of hydrogen. the h is capitalized because it is the hydrogen element symbol. Although the ph of pure water is 7, drinking water and natural water exhibits a ph range because it contains dissolved minerals and gases. The range goes from 0 to 14, with 7 being. What Does Ph Of Water Stand For.
From blog.havells.com
Right pH Level in Drinking Water How Essential? Havells India Blog What Does Ph Of Water Stand For Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph of 7 is considered neutral (e.g., pure water). Phs of less than 7 indicate acidity, whereas a ph of greater than 7 indicates a base. Ph is a measure of how acidic/basic water is. The ph scale shows the leaves of acidity and alkalinity of. What Does Ph Of Water Stand For.