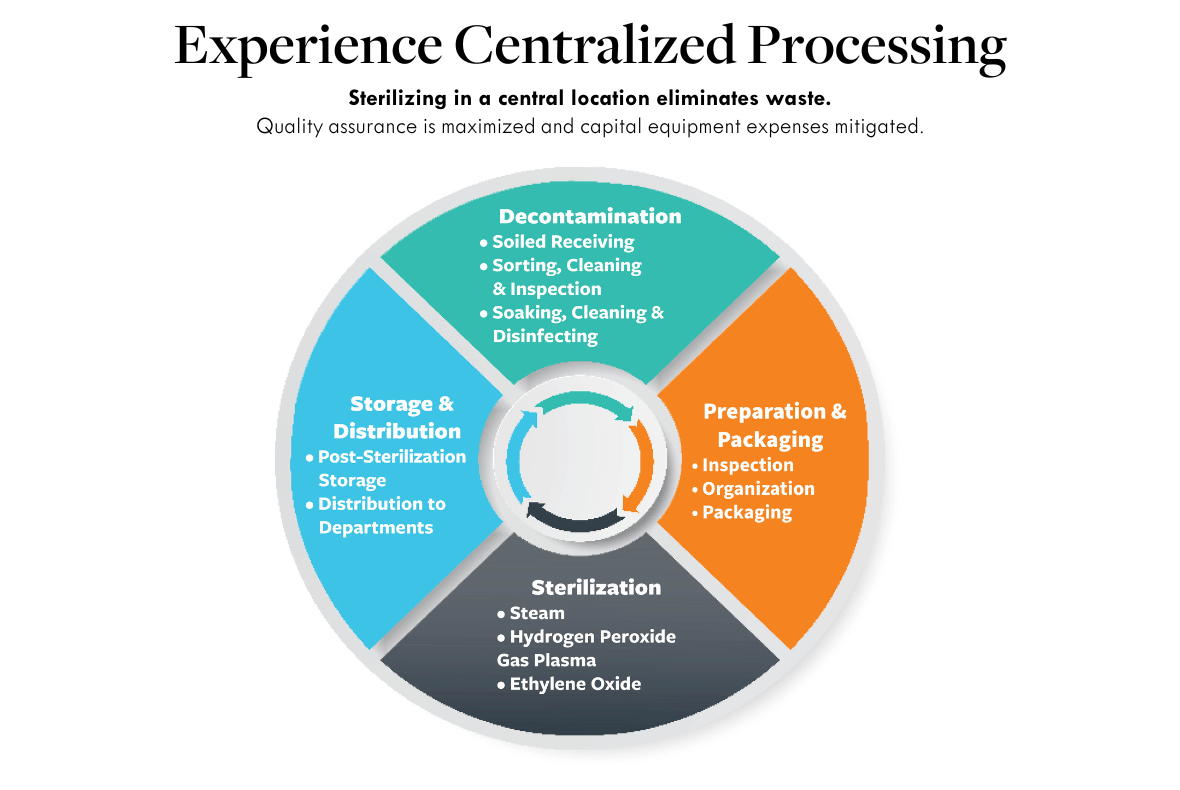
Quality Assurance In Sterile Processing . Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department.
from www.crothall.com
The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and.
Learn More About Sterile Processing Services Crothall Healthcare
Quality Assurance In Sterile Processing Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and.
From www.burkhartdental.com
Sterilization Monitoring An Important Quality Assurance Process Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Quality Assurance In Sterile Processing Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Anagement system. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Quality Assurance In Sterile Processing Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators. Quality Assurance In Sterile Processing.
From capstone.isye.gatech.edu
Sterile Processing Department Quality Assurance and Resource Allocation Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Quality assurance in sterile processing is. Quality Assurance In Sterile Processing.
From kerone.com
Different Types of Sterilization Process Quality Assurance In Sterile Processing The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile processing department hires stock photography and images Alamy Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Unlike other “processing” fields. Quality Assurance In Sterile Processing.
From cssdtechnicianhub.com
sterileprocessingqualityassurance CSSD Technician Hub Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Ensuring consistency of sterilization practices requires a comprehensive program. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT 3M™ Attest™ Sterile U Meeting March 19, 2009 PowerPoint Quality Assurance In Sterile Processing Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Sterility assurance products including biological. Quality Assurance In Sterile Processing.
From www.researchgate.net
(PDF) Quality Assurance for Sterile Products Quality Assurance In Sterile Processing Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The three articles in this special focus issue discuss vastly distinct. Quality Assurance In Sterile Processing.
From wecarepharm.com
Quality Assurance WeCare Pharmacy Quality Assurance In Sterile Processing The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Sterility. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Quality Assurance In Sterile Processing The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Quality. Quality Assurance In Sterile Processing.
From www.researchgate.net
(PDF) Quality Assurance for Sterile Products Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Unlike other. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Quality Assurance In Sterile Processing Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Ensuring consistency of sterilization. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile processing department hires stock photography and images Alamy Quality Assurance In Sterile Processing Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Unlike other “processing” fields which work on a batch or assembly line, sterile. Quality Assurance In Sterile Processing.
From www.docseducation.com
Sterilization Monitoring; An Important Quality Assurance Process DOCS Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Ensuring consistency of sterilization practices requires a comprehensive. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile processing department hires stock photography and images Alamy Quality Assurance In Sterile Processing Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Sterility assurance products including biological indicators (bi). Quality Assurance In Sterile Processing.
From berkshiresterilemanufacturing.com
How pulsed light technology provides added sterility assurance in Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Sterility assurance products including biological indicators (bi) and chemical indicators. Quality Assurance In Sterile Processing.
From dokumen.tips
(PDF) QUALITY ASSURANCE IN STERILIZATION · routine control of a Quality Assurance In Sterile Processing Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Sterility assurance products including biological indicators (bi) and. Quality Assurance In Sterile Processing.
From healthcare.seattlecentral.edu
Sterile Processing Healthcare Programs Quality Assurance In Sterile Processing Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Anagement system (qms) for processed medical devices and loaned instruments, as. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile processing department hires stock photography and images Alamy Quality Assurance In Sterile Processing Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Ensuring consistency of sterilization. Quality Assurance In Sterile Processing.
From pharmaguidances.com
STERILITY ASSURANCE Quality Assurance In Sterile Processing The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Unlike other “processing” fields which work on a batch or assembly line,. Quality Assurance In Sterile Processing.
From www.researchgate.net
(PDF) Sterile processing quality assurance and validation Quality Assurance In Sterile Processing Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The association for the advancement. Quality Assurance In Sterile Processing.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Quality Assurance In Sterile Processing The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile processing department hires stock photography and images Alamy Quality Assurance In Sterile Processing Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Ensuring. Quality Assurance In Sterile Processing.
From myhspa.org
Quality Assurance in Healthcare Facilites / Recall Policy Healthcare Quality Assurance In Sterile Processing Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Sterility assurance products including biological indicators (bi) and chemical. Quality Assurance In Sterile Processing.
From www.casemed.com
"Managing Sterile Processing" Quality Assurance In Sterile Processing Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. Anagement system (qms) for processed medical devices and loaned instruments, as. Quality Assurance In Sterile Processing.
From myhspa.org
Establishing a Process for Periodic Product Quality Assurance Testing Quality Assurance In Sterile Processing Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Anagement system (qms) for processed medical devices and loaned instruments, as well as. Quality Assurance In Sterile Processing.
From www.burkhartdental.com
Sterilization Monitoring An Important Quality Assurance Process Quality Assurance In Sterile Processing The association for the advancement of medical instrumentation (aami) guidelines state that sterile processing personnel should. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Assessments help to set polices, implement necessary. Quality Assurance In Sterile Processing.
From www.crothall.com
Learn More About Sterile Processing Services Crothall Healthcare Quality Assurance In Sterile Processing Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance.. Quality Assurance In Sterile Processing.
From cssdtechnicianhub.com
Central Sterile Processing and Distribution CSSD Technician Hub Quality Assurance In Sterile Processing Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Ensuring. Quality Assurance In Sterile Processing.
From cmo.fresenius-kabi.com
Our Sterile Pharmaceutical Technologies Fresenius Kabi Contract Quality Assurance In Sterile Processing Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Sterility assurance products. Quality Assurance In Sterile Processing.
From pharmacyconnection.ca
Focus on Quality Assurance Tips on Meeting Sterile Compounding Quality Assurance In Sterile Processing Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. The. Quality Assurance In Sterile Processing.
From www.alamy.com
Sterile Processing High Resolution Stock Photography and Images Alamy Quality Assurance In Sterile Processing Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Assessments help. Quality Assurance In Sterile Processing.
From app.docseducation.com
Sterilization Monitoring; An Important Quality Assurance Process DOCS Quality Assurance In Sterile Processing Ensuring consistency of sterilization practices requires a comprehensive program that ensures operator competence and. The three articles in this special focus issue discuss vastly distinct aspects of sterile processing, including water quality and quantity, benchmarking spd. Assessments help to set polices, implement necessary quality assurance (qa) tests, and ensure practices promote the best performance. The association for the advancement of. Quality Assurance In Sterile Processing.
From mms.mckesson.com
Sterilization packaging & quality assurance McKesson MedicalSurgical Quality Assurance In Sterile Processing Anagement system (qms) for processed medical devices and loaned instruments, as well as to prevent adverse patient events. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Unlike other “processing” fields which work on a batch or assembly line, sterile processing is a quality assurance department.. Quality Assurance In Sterile Processing.