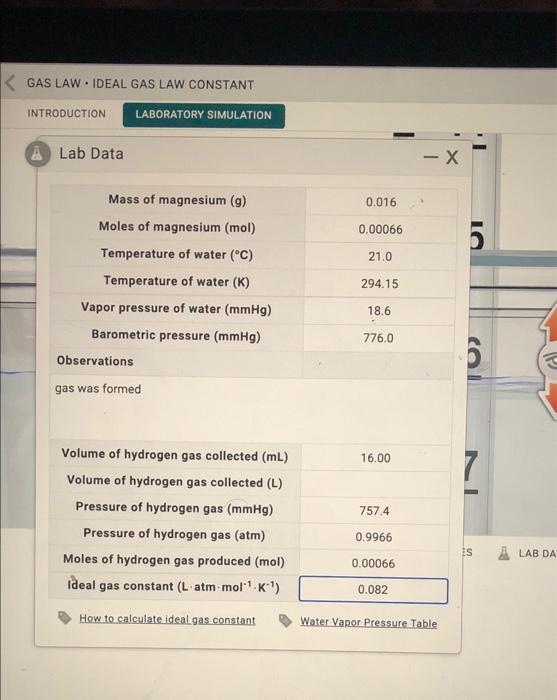
Determination Of Ideal Gas Law Constant Lab Report . The ideal gas law arises from several different gas laws. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This document outlines an experiment to determine the ideal gas law constant through trials measuring. Experimental determination of the gas constant purpose: Determination of the gas constant, r. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. If your concentration, moles, or r calculations are. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Determination of ideal gas law constant. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\).
from www.chegg.com
The objectives of this lab are to experimentally determine the value of the gas constant, r, and. If your concentration, moles, or r calculations are. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). This document outlines an experiment to determine the ideal gas law constant through trials measuring. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Determination of the gas constant, r. Experimental determination of the gas constant purpose: The ideal gas law arises from several different gas laws. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you.
Solved K GAS LAW IDEAL GAS LAW CONSTANT INTRODUCTION
Determination Of Ideal Gas Law Constant Lab Report This document outlines an experiment to determine the ideal gas law constant through trials measuring. Determination of ideal gas law constant. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. If your concentration, moles, or r calculations are. The ideal gas law arises from several different gas laws. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. Determination of the gas constant, r. Experimental determination of the gas constant purpose: In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). This document outlines an experiment to determine the ideal gas law constant through trials measuring.
From www.studocu.com
Lab 9 Evaluation of the Gas Law Constant CHEM 1411 LSC Studocu Determination Of Ideal Gas Law Constant Lab Report Experimental determination of the gas constant purpose: Determination of the gas constant, r. The ideal gas law arises from several different gas laws. If your concentration, moles, or r calculations are. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). The gas inside. Determination Of Ideal Gas Law Constant Lab Report.
From www.yumpu.com
Lab 12 The Ideal Gas Law Determination Of Ideal Gas Law Constant Lab Report Determination of the gas constant, r. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. This lab will test the relationship between pressure and the number. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
11 Ideal Gas Law Constant ADA CHEMISTRY Determination of Ideal Gas Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The ideal gas law arises from several different gas laws. Experimental determination of the gas constant purpose: Determination of the gas constant, r. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Solved GAS LAW IDEAL GAS LAW CONSTANT INTRODUCTION Determination Of Ideal Gas Law Constant Lab Report In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. This lab will test the relationship between pressure and the number of moles of gas as described by the. Determination Of Ideal Gas Law Constant Lab Report.
From studylib.net
LAB PRACTICE 1 IDEAL GAS CONSTANT R worksheet Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Determination of the gas constant, r. The ideal gas law arises from several different gas laws. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. This lab will test the relationship between pressure and the number of. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
Determination of Ideal Gas Law Constant Lab Report Determination of Determination Of Ideal Gas Law Constant Lab Report Determination of the gas constant, r. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). Experimental determination of the gas constant purpose: Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. This document outlines an experiment to. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Determination of ideal gas law constant pre lab Data Studypool Determination Of Ideal Gas Law Constant Lab Report Experimental determination of the gas constant purpose: In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. If your. Determination Of Ideal Gas Law Constant Lab Report.
From www.scribd.com
Chem Lab Report 9 (2)Gas Law Determination Of Ideal Gas Law Constant Lab Report If your concentration, moles, or r calculations are. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Determination of ideal gas law constant. This document outlines an experiment to determine the ideal gas law constant through trials measuring. The objectives of this lab are to experimentally determine the value of the gas. Determination Of Ideal Gas Law Constant Lab Report.
From www.studyxapp.com
chemistry ideal gas law constant introduction laboratory simulation a Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Determination of ideal gas law constant. If your concentration, moles, or r calculations are. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Experimental determination of the gas constant purpose: Determination of the gas constant, r. This. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
M7 Lab Determination of Ideal Gas Law Constant Determination of Ideal Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Determination of ideal gas law constant. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Determination of ideal gas law constant. Lab report Studypool Determination Of Ideal Gas Law Constant Lab Report If your concentration, moles, or r calculations are. The ideal gas law arises from several different gas laws. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Determination of ideal gas law constant. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This document. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Determination of R The Gas Law Constant Report Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This document outlines an experiment to determine the ideal gas law. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Ideal Gas Lab Report Studypool Determination Of Ideal Gas Law Constant Lab Report The objectives of this lab are to experimentally determine the value of the gas constant, r, and. The ideal gas law arises from several different gas laws. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Boyle’s law describes the inverse relationship between pressure. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Chemistry 101 lab report universal gas constant Studypool Determination Of Ideal Gas Law Constant Lab Report The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Experimental determination of the gas constant purpose: In this lab, students will measure various properties of a sample. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
6.1 lab notes and coursework Determination of Ideal Gas Law Constant Determination Of Ideal Gas Law Constant Lab Report The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The ideal gas law arises from several different gas laws. This lab will test the relationship between pressure and the number of moles of gas as described by. Determination Of Ideal Gas Law Constant Lab Report.
From studylib.net
Experiment 6 Experimental Determination of Ideal Gas Law Constant Determination Of Ideal Gas Law Constant Lab Report The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). The objectives. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Determination of ideal gas law constant. Lab report Studypool Determination Of Ideal Gas Law Constant Lab Report This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The ideal gas law arises from several different gas laws. Determination of the gas constant, r. Determination of ideal gas. Determination Of Ideal Gas Law Constant Lab Report.
From www.pdffiller.com
Fillable Online EXPERIMENT 18 DETERMINATION OF THE IDEAL GAS LAW Determination Of Ideal Gas Law Constant Lab Report This document outlines an experiment to determine the ideal gas law constant through trials measuring. The ideal gas law arises from several different gas laws. Determination of ideal gas law constant. Determination of the gas constant, r. If your concentration, moles, or r calculations are. The gas inside the tire has a volume of 20.00 l at a pressure of. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Solved REPORT SHEET Determination of R The Gas Law Constant Determination Of Ideal Gas Law Constant Lab Report This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. This document outlines an experiment to determine the ideal gas law constant through trials measuring. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The ideal gas law arises. Determination Of Ideal Gas Law Constant Lab Report.
From chem.libretexts.org
10 Experimental Determination of the Gas Constant (Experiment Determination Of Ideal Gas Law Constant Lab Report If your concentration, moles, or r calculations are. Experimental determination of the gas constant purpose: This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. The ideal gas law arises from several different gas laws. The gas inside the tire has a volume of 20.00. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Solved CHEMISTRY. IDEAL GAS LAW CONSTANT INTRODUCTION Determination Of Ideal Gas Law Constant Lab Report The ideal gas law arises from several different gas laws. This document outlines an experiment to determine the ideal gas law constant through trials measuring. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). Determination of ideal gas law constant. This lab will. Determination Of Ideal Gas Law Constant Lab Report.
From answerhappy.com
DETERMINATION OF THE GAS CONSTANT INTRODUCTION The ideal gas law, PV Determination Of Ideal Gas Law Constant Lab Report In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. Experimental determination of the gas constant purpose: If your concentration, moles, or r calculations are. This lab. Determination Of Ideal Gas Law Constant Lab Report.
From studylib.net
ideal gas law lab Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. This document outlines an experiment to determine the ideal gas law constant through trials measuring. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). This lab will test. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Solved K GAS LAW IDEAL GAS LAW CONSTANT INTRODUCTION Determination Of Ideal Gas Law Constant Lab Report The ideal gas law arises from several different gas laws. Determination of the gas constant, r. Experimental determination of the gas constant purpose: This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Determination of ideal gas law constant. In this lab, students will measure. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Determination of Ideal Gas Law Constant Lab. (I Determination Of Ideal Gas Law Constant Lab Report The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This document outlines an experiment to determine the ideal gas law constant through trials measuring. Experimental determination of the gas constant purpose: The ideal gas law arises from several different gas laws. The gas inside the tire has a volume of 20.00 l. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
CHM 101L M6 Ideal Gas Law Constant Lab Report Determination of Ideal Determination Of Ideal Gas Law Constant Lab Report The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. Determination of ideal gas law constant. Boyle’s law describes the inverse relationship between pressure and volume, p ∝. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
REPORT SHEET Determination of R The Gas Law Constant Determination Of Ideal Gas Law Constant Lab Report The objectives of this lab are to experimentally determine the value of the gas constant, r, and. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas constant, \(r\). The ideal gas law arises from several different gas laws. This lab will test the relationship between. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
Course HERO Ideal Gas Law Constant Lab Report Determination of Ideal Determination Of Ideal Gas Law Constant Lab Report The ideal gas law arises from several different gas laws. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Determination of the gas constant, r. Experimental determination of the gas constant purpose: This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law. Determination Of Ideal Gas Law Constant Lab Report.
From www.studypool.com
SOLUTION Determination of ideal gas law constant. Lab report Studypool Determination Of Ideal Gas Law Constant Lab Report If your concentration, moles, or r calculations are. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. Determination of ideal gas law constant. Determination of the gas. Determination Of Ideal Gas Law Constant Lab Report.
From sciencenotes.org
Ideal Gas Law Formula and Examples Determination Of Ideal Gas Law Constant Lab Report If your concentration, moles, or r calculations are. Experimental determination of the gas constant purpose: Determination of ideal gas law constant. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. This document outlines an experiment to determine. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
CHM 101L M6 Ideal Gas Law Constant Lab Report Determination of Ideal Determination Of Ideal Gas Law Constant Lab Report Determination of ideal gas law constant. Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Determination of the gas constant, r. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. This document outlines an experiment to determine the ideal gas law constant through trials measuring. The. Determination Of Ideal Gas Law Constant Lab Report.
From www.chegg.com
Determination of Ideal Gas Law Constant Lab. (I Determination Of Ideal Gas Law Constant Lab Report This document outlines an experiment to determine the ideal gas law constant through trials measuring. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. Determination of ideal gas law constant. This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
Lab Report Determination of Ideal Gas Law Constant Studocu Determination Of Ideal Gas Law Constant Lab Report This lab will test the relationship between pressure and the number of moles of gas as described by the ideal gas law and have you. The objectives of this lab are to experimentally determine the value of the gas constant, r, and. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally. Determination Of Ideal Gas Law Constant Lab Report.
From www.studocu.com
Lab 11 Determination of Ideal Gas Law Constant 2.pdf Determination of Determination Of Ideal Gas Law Constant Lab Report Boyle’s law describes the inverse relationship between pressure and volume, p ∝ 1/v, for a. Experimental determination of the gas constant purpose: The gas inside the tire has a volume of 20.00 l at a pressure of 5.00 atm. The ideal gas law arises from several different gas laws. Determination of the gas constant, r. This document outlines an experiment. Determination Of Ideal Gas Law Constant Lab Report.