Iron Element Number Of Neutrons . The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Find out how iron forms compounds in different oxidation states and what are its common alloys. Iron (fe) has an atomic mass of 26. The number of protons determines the element, but the number of neutrons in the. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron is a chemical element; Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It has the symbol fe (from latin ferrum 'iron') and atomic number 26.
from material-properties.org
Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron is a chemical element; It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Find out how iron forms compounds in different oxidation states and what are its common alloys. Iron (fe) has an atomic mass of 26. The number of protons determines the element, but the number of neutrons in the. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay.
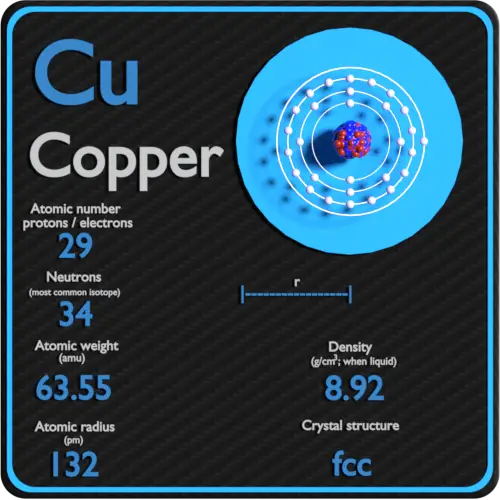
Copper Periodic Table and Atomic Properties
Iron Element Number Of Neutrons Find out how iron forms compounds in different oxidation states and what are its common alloys. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Find out how iron forms compounds in different oxidation states and what are its common alloys. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron is a chemical element; Iron (fe) has an atomic mass of 26. The number of protons determines the element, but the number of neutrons in the. It has the symbol fe (from latin ferrum 'iron') and atomic number 26.
From www.teachoo.com
Neutron Discovery, Difference and more Teachoo Concepts Iron Element Number Of Neutrons 1535.0 °c (1808.15 k, 2795.0 °f) boiling. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. 112 rows protons neutrons & electrons of all elements (list + images) september. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Iron (fe) has an atomic mass of 26. Iron is a chemical element; The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Find out how iron forms compounds in different oxidation states and. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons 1535.0 °c (1808.15 k, 2795.0 °f) boiling. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Iron is a chemical element; It is a metal that belongs to the first transition series and group 8. Iron Element Number Of Neutrons.
From quizuphillward.z4.web.core.windows.net
What Element Has 7 Protons And 7 Electrons Iron Element Number Of Neutrons Iron is a chemical element; 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Find out how iron forms compounds in different oxidation states and what are its common alloys. The number of protons determines the element, but the number of neutrons in the. Learn about the number of protons, neutrons, and. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Element Number Of Neutrons The number of protons determines the element, but the number of neutrons in the. It is a metal that belongs to the first transition series and group 8 of the periodic table. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron (fe) has an atomic mass of 26. Iron is a chemical element; 112. Iron Element Number Of Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It is a metal that belongs. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. The number of protons determines the element, but the number of neutrons in the. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It is a metal that belongs to the first transition series. Iron Element Number Of Neutrons.
From jcrxpetxmb.blogspot.com
How To Find How Many Neutrons Are In An Element The atomic number of Iron Element Number Of Neutrons It is a metal that belongs to the first transition series and group 8 of the periodic table. Find out how iron forms compounds in different oxidation states and what are its common alloys. Iron (fe) has an atomic mass of 26. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. Iron is a chemical element with atomic number 26 which means. Iron Element Number Of Neutrons.
From awesomehome.co
Periodic Table Iron Number Of Neutrons Awesome Home Iron Element Number Of Neutrons Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. The number of protons determines the element, but the number of neutrons in the. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron is a chemical element with atomic number 26 which means. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons Iron is a chemical element; 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Iron (fe) has an atomic mass of 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. Iron is a chemical element with atomic number 26. Iron Element Number Of Neutrons.
From awesomehome.co
Understanding The Periodic Table Protons Neutrons And Electrons Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. 1535.0 °c (1808.15 k, 2795.0 °f). Iron Element Number Of Neutrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Element Number Of Neutrons Find out how iron forms compounds in different oxidation states and what are its common alloys. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. It is a metal that belongs to the first transition. Iron Element Number Of Neutrons.
From jaelynn-has-rodriguez.blogspot.com
Of the Following Which Element Has the Most Protons JaelynnhasRodriguez Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. The number of protons determines the element, but the number of neutrons in the. Find out how iron forms compounds in different oxidation states and what are its common alloys. The mass number represents the number of protons plus. Iron Element Number Of Neutrons.
From www.youtube.com
How to find the Protons Neutrons and Electrons of an element on the Iron Element Number Of Neutrons It is a metal that belongs to the first transition series and group 8 of the periodic table. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Iron is a chemical element; Find. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons The number of protons determines the element, but the number of neutrons in the. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. Learn about the number of protons, neutrons, and. Iron Element Number Of Neutrons.
From www.numerade.com
SOLVED With the help of the periodic table, complete the following Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron (fe) has an atomic mass of 26. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. The number of protons determines the. Iron Element Number Of Neutrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Element Number Of Neutrons Iron is a chemical element; 1535.0 °c (1808.15 k, 2795.0 °f) boiling. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic.. Iron Element Number Of Neutrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Element Number Of Neutrons The number of protons determines the element, but the number of neutrons in the. Find out how iron forms compounds in different oxidation states and what are its common alloys. It is a metal that belongs to the first transition series and group 8 of the periodic table. 112 rows protons neutrons & electrons of all elements (list + images). Iron Element Number Of Neutrons.
From redesigngreece.blogspot.com
How To Find The Number Of Neutrons In An Element redesigngreece Iron Element Number Of Neutrons 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron is a chemical element; The number of protons determines. Iron Element Number Of Neutrons.
From www.baamboozle.com
Atomic Structure and Elements Baamboozle Baamboozle The Most Fun Iron Element Number Of Neutrons The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. It is a metal that belongs to the first transition series and group 8 of the periodic table. Iron (fe). Iron Element Number Of Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It is a metal that belongs to the first transition series and. Iron Element Number Of Neutrons.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Iron Element Number Of Neutrons Iron (fe) has an atomic mass of 26. Iron is a chemical element; It has the symbol fe (from latin ferrum 'iron') and atomic number 26. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. The number of protons determines the element, but the number of neutrons in the. Learn about the number of protons, neutrons, and electrons in iron, a metal. Iron Element Number Of Neutrons.
From material-properties.org
Copper Periodic Table and Atomic Properties Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. The number of protons determines the element, but the number of neutrons in the. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. It is a metal that belongs to the first transition series and. Iron Element Number Of Neutrons.
From studylib.net
atom Iron Element Number Of Neutrons Iron (fe) has an atomic mass of 26. Iron is a chemical element; The number of protons determines the element, but the number of neutrons in the. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. 1535.0 °c. Iron Element Number Of Neutrons.
From brainly.ph
Activity 2.3Direction Given the Atomic number and mass number of the Iron Element Number Of Neutrons It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Find out how iron forms compounds in different oxidation states and what are its common alloys. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons. Iron Element Number Of Neutrons.
From hxeuoftlg.blob.core.windows.net
Iron Element Neutrons at Cristina Armstrong blog Iron Element Number Of Neutrons Iron is a chemical element; The number of protons determines the element, but the number of neutrons in the. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. The mass number represents the number of protons plus neutrons in. Iron Element Number Of Neutrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Element Number Of Neutrons The number of protons determines the element, but the number of neutrons in the. Iron (fe) has an atomic mass of 26. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. It is a metal. Iron Element Number Of Neutrons.
From sciencing.com
How to Find the Neutrons in the Periodic Table Sciencing Iron Element Number Of Neutrons The number of protons determines the element, but the number of neutrons in the. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The mass number represents the number of protons plus neutrons in. Iron Element Number Of Neutrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Sulfur (S, S2) Iron Element Number Of Neutrons Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The number of protons determines the element, but the number of neutrons in the. 1535.0 °c (1808.15 k, 2795.0 °f) boiling. It has the symbol. Iron Element Number Of Neutrons.
From variosmateriais.blogspot.com
Journal Of Materials Science Materials In Medicine Abbreviation Iron Element Number Of Neutrons 1535.0 °c (1808.15 k, 2795.0 °f) boiling. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Iron is a chemical element; It is a metal that belongs to the first transition series and group 8 of the periodic table. Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26. Iron Element Number Of Neutrons.
From chemistry291.blogspot.com
How Many Neutrons Does Iron(Fe) Have?Number of Neutrons in Iron(Fe) Iron Element Number Of Neutrons Find out how iron forms compounds in different oxidation states and what are its common alloys. The number of protons determines the element, but the number of neutrons in the. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. It has the symbol fe (from latin ferrum 'iron') and atomic number 26.. Iron Element Number Of Neutrons.
From reviewhomedecor.co
Periodic Table Iron Number Of Neutrons Review Home Decor Iron Element Number Of Neutrons Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron is a chemical element; The number of protons determines the element, but the number of neutrons in the. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. It has the symbol fe (from latin ferrum. Iron Element Number Of Neutrons.
From brainly.in
List of elements and their number of protons neutrons and electrons Iron Element Number Of Neutrons Learn about the number of protons, neutrons, and electrons in iron, a metal with atomic number 26 and four stable isotopes. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Iron (fe) has an atomic mass of 26. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Iron is a chemical. Iron Element Number Of Neutrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Element Number Of Neutrons 1535.0 °c (1808.15 k, 2795.0 °f) boiling. The number of protons determines the element, but the number of neutrons in the. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. The mass number represents the. Iron Element Number Of Neutrons.
From www.nagwa.com
Question Video Calculating the Number of Neutrons in an Atom of Iron Element Number Of Neutrons 1535.0 °c (1808.15 k, 2795.0 °f) boiling. 112 rows protons neutrons & electrons of all elements (list + images) september 1, 2024 by jay. It has the symbol fe (from latin ferrum 'iron') and atomic number 26. Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic. The number. Iron Element Number Of Neutrons.