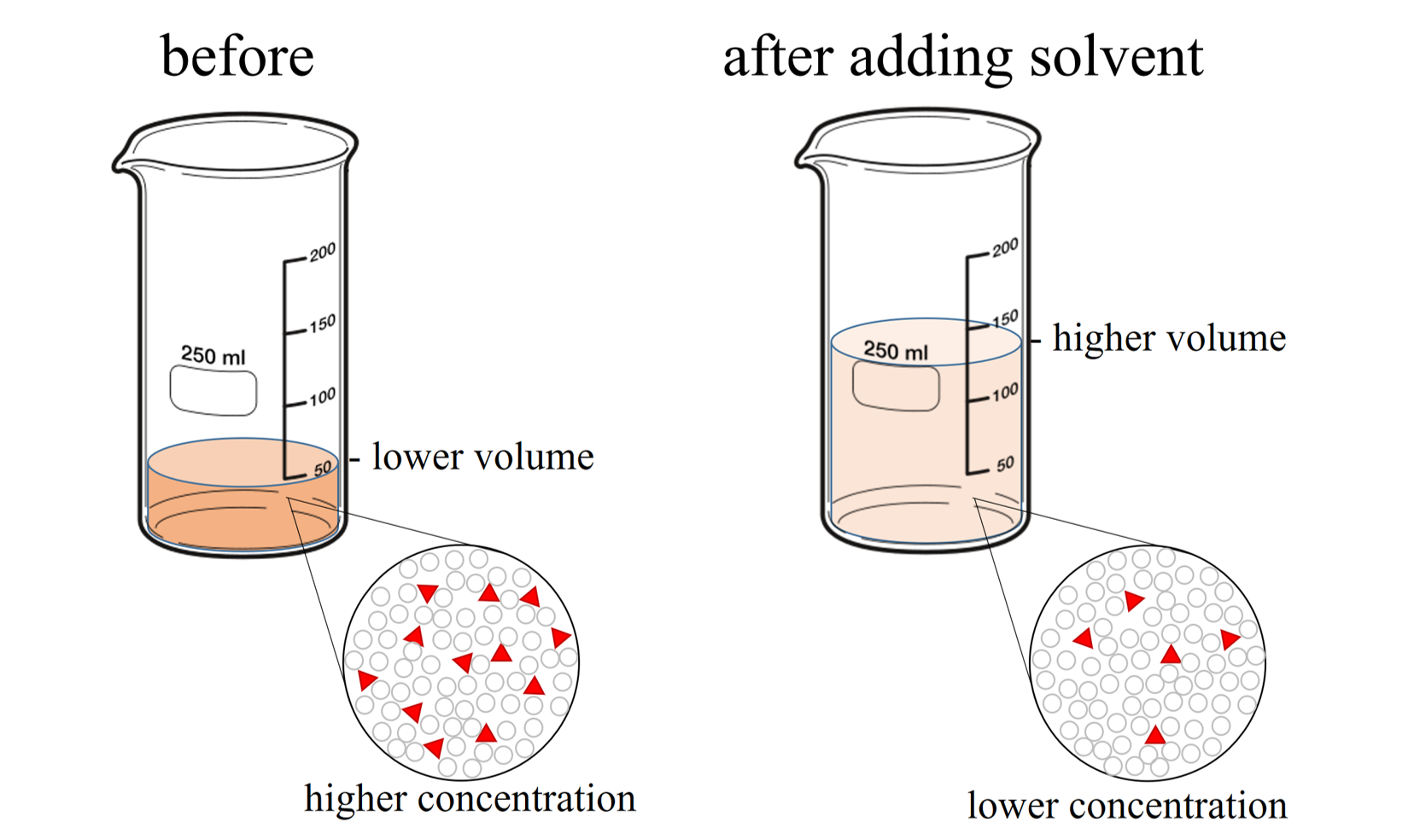
Dilution Chem Worksheet 15-5 . This is accomplished by adding more solvent. _____ perform the following dilutions calculation. More chemistry tutorials and practice can be found at www.chemfiesta.com. A solution can be made less concentrated in a process called dilution. Since only the volume changes in this solution, this same. Multiply the molarity and volume of the first solution to obtain moles of solute. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name:
from chem.libretexts.org
Multiply the molarity and volume of the first solution to obtain moles of solute. More chemistry tutorials and practice can be found at www.chemfiesta.com. A solution can be made less concentrated in a process called dilution. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. This is accomplished by adding more solvent. I add 560 ml more water to it? Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. _____ perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?
14.7 Solution Dilution Chemistry LibreTexts
Dilution Chem Worksheet 15-5 A solution can be made less concentrated in a process called dilution. This is accomplished by adding more solvent. A solution can be made less concentrated in a process called dilution. I add 560 ml more water to it? Multiply the molarity and volume of the first solution to obtain moles of solute. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? _____ perform the following dilutions calculation. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: Since only the volume changes in this solution, this same. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. More chemistry tutorials and practice can be found at www.chemfiesta.com.
From studyele1metw.z21.web.core.windows.net
The Mole Concept Worksheets Dilution Chem Worksheet 15-5 More chemistry tutorials and practice can be found at www.chemfiesta.com. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? A solution can be made less concentrated in a process called dilution. I add 560 ml more water to it? Since only the volume. Dilution Chem Worksheet 15-5.
From studylib.net
Dilution Worksheet Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. This is accomplished by adding more solvent. Since only the volume changes in this solution, this same. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Dilution Chem Worksheet 15-5.
From www.learnsci.com
LearnSci Smart Worksheet Serial Dilutions Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? More chemistry tutorials and practice can be found at www.chemfiesta.com. I add 560 ml more water to it? This is accomplished by adding more solvent. Multiply the molarity and volume of the first solution. Dilution Chem Worksheet 15-5.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chem Worksheet 15-5 A solution can be made less concentrated in a process called dilution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. _____ perform the following dilutions calculation. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: Solution mi x v, =mol w.ter conceniraied solution is diluiej with more. Dilution Chem Worksheet 15-5.
From studylib.net
Dilution Dilution Chem Worksheet 15-5 I add 560 ml more water to it? More chemistry tutorials and practice can be found at www.chemfiesta.com. This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Dilutions worksheet i revised sunday, march 24, 2013 1. Dilution Chem Worksheet 15-5.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Chem Worksheet 15-5 This is accomplished by adding more solvent. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. More chemistry tutorials and practice can be found at www.chemfiesta.com. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: Since only the volume changes in this solution, this same. A solution can. Dilution Chem Worksheet 15-5.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. A solution can be made less concentrated in a process called dilution. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. Since only the volume changes in this solution, this same. Multiply the molarity and volume of the. Dilution Chem Worksheet 15-5.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: More chemistry tutorials and practice can be found at. Dilution Chem Worksheet 15-5.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Dilution Chem Worksheet 15-5 Since only the volume changes in this solution, this same. I add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: _____ perform the following. Dilution Chem Worksheet 15-5.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Chem Worksheet 15-5 A solution can be made less concentrated in a process called dilution. _____ perform the following dilutions calculation. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i dilute. Dilution Chem Worksheet 15-5.
From printablemagicdrescher.z19.web.core.windows.net
Concentration And Dilution Worksheet Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of. Dilution Chem Worksheet 15-5.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Chem Worksheet 15-5 More chemistry tutorials and practice can be found at www.chemfiesta.com. _____ perform the following dilutions calculation. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. I add 560 ml more water to it? Multiply the molarity and volume of the first solution to obtain moles of solute. This is accomplished by adding more solvent. Dilutions worksheet. Dilution Chem Worksheet 15-5.
From lessoncampusunspelt.z13.web.core.windows.net
Molarity By Dilution Worksheet Dilution Chem Worksheet 15-5 I add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. _____ perform the following dilutions calculation. Multiply the molarity and volume of the first solution to obtain moles of solute. Since only the volume changes in. Dilution Chem Worksheet 15-5.
From pngmusson.blogspot.com
Dilution Calculations Worksheet 2 / Png Musson Dilution Chem Worksheet 15-5 I add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to. Dilution Chem Worksheet 15-5.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Chem Worksheet 15-5 Multiply the molarity and volume of the first solution to obtain moles of solute. More chemistry tutorials and practice can be found at www.chemfiesta.com. A solution can be made less concentrated in a process called dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Dilution Chem Worksheet 15-5.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. I add 560 ml more water to it? Multiply the molarity and volume of the first solution to obtain moles of solute. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: This is. Dilution Chem Worksheet 15-5.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chem Worksheet 15-5 Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. _____ perform the following dilutions calculation. I add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of. Dilution Chem Worksheet 15-5.
From giovyrtvh.blob.core.windows.net
Dilution Integrity Vs Dilution Linearity at Karen Zambrano blog Dilution Chem Worksheet 15-5 Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? _____ perform the following dilutions calculation. Multiply the molarity and volume of the first solution to obtain moles of solute. More chemistry. Dilution Chem Worksheet 15-5.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. Since only the volume changes in this solution, this same. Dilutions worksheet i revised sunday, march. Dilution Chem Worksheet 15-5.
From lessonmagicnunez.z21.web.core.windows.net
Molarity Dilution Worksheets Dilution Chem Worksheet 15-5 More chemistry tutorials and practice can be found at www.chemfiesta.com. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 2) if i dilute 250 ml of 0.10 m lithium acetate solution. Dilution Chem Worksheet 15-5.
From studylib.net
Dilutions Worksheet 11UChem Dilution Chem Worksheet 15-5 I add 560 ml more water to it? Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Since only the volume changes in this. Dilution Chem Worksheet 15-5.
From worksheets.clipart-library.com
Dilutions Worksheet Worksheets Library Dilution Chem Worksheet 15-5 Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: I add 560 ml more water to it? 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. Multiply the molarity and volume of the first solution to obtain moles of solute. A solution can be made less concentrated in. Dilution Chem Worksheet 15-5.
From hxepnhstr.blob.core.windows.net
Dilution Equation Sample at Christy Burk blog Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. More chemistry tutorials and practice can be found at www.chemfiesta.com. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution. Dilution Chem Worksheet 15-5.
From learninglibrarylinton.z21.web.core.windows.net
Dilution Practice Problems Worksheet Answers Dilution Chem Worksheet 15-5 Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. I add 560 ml more water to it? Since only the volume changes in this solution, this same. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. _____ perform the following dilutions calculation. This is accomplished by adding. Dilution Chem Worksheet 15-5.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution Chem Worksheet 15-5 Multiply the molarity and volume of the first solution to obtain moles of solute. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. I add 560 ml more water to it? More chemistry tutorials and practice can be found at www.chemfiesta.com. A solution. Dilution Chem Worksheet 15-5.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Chem Worksheet 15-5 Multiply the molarity and volume of the first solution to obtain moles of solute. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: _____ perform the following dilutions calculation. 1) if i add 25 ml of water to 125. Dilution Chem Worksheet 15-5.
From learningisidro.z13.web.core.windows.net
Making Dilutions Worksheets Dilution Chem Worksheet 15-5 More chemistry tutorials and practice can be found at www.chemfiesta.com. This is accomplished by adding more solvent. Multiply the molarity and volume of the first solution to obtain moles of solute. A solution can be made less concentrated in a process called dilution. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. I add 560 ml. Dilution Chem Worksheet 15-5.
From printablemediaells.z21.web.core.windows.net
Dilution Worksheet Chemistry Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. Multiply the molarity and volume of the first solution to obtain moles of solute. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. More chemistry tutorials and practice can be found at www.chemfiesta.com.. Dilution Chem Worksheet 15-5.
From printablelibswathed.z19.web.core.windows.net
Molarity And Dilution Worksheet Dilution Chem Worksheet 15-5 More chemistry tutorials and practice can be found at www.chemfiesta.com. _____ perform the following dilutions calculation. Multiply the molarity and volume of the first solution to obtain moles of solute. A solution can be made less concentrated in a process called dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what. Dilution Chem Worksheet 15-5.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? _____ perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 2) if. Dilution Chem Worksheet 15-5.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Chem Worksheet 15-5 A solution can be made less concentrated in a process called dilution. I add 560 ml more water to it? Since only the volume changes in this solution, this same. _____ perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Dilution Chem Worksheet 15-5.
From learningmedialudovic.z21.web.core.windows.net
Converting Units Of Pressure Worksheets Dilution Chem Worksheet 15-5 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. This is accomplished by adding more solvent. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a. Dilution Chem Worksheet 15-5.
From www.scribd.com
Dilutions Worksheet Key For Chemistry PDF Dilution Chem Worksheet 15-5 _____ perform the following dilutions calculation. This is accomplished by adding more solvent. Solution mi x v, =mol w.ter conceniraied solution is diluiej with more solvent. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of. Dilution Chem Worksheet 15-5.
From www.studocu.com
Dilutions Worksheet Dilution problems CHEM 1010 Studocu Dilution Chem Worksheet 15-5 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml,. Dilutions worksheet i revised sunday, march 24, 2013 1 revision 1.0 name: More chemistry tutorials and practice can be found at www.chemfiesta.com. I add 560 ml more water to it? Multiply the molarity and volume of the first solution to obtain moles. Dilution Chem Worksheet 15-5.