Titration Lab Setup . As the addition takes place, the two reagents in. This video takes you through the proper technique for setting up and performing a titration. For instance, you might add a standard base solution to an mystery acid solution. Valencia college lab technique 22: The buret is held in place by the buret clamp, which is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. This is the first video in a two part. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Swirl the water around a few times before opening the stopcock and allowing it to drain. [2] repeat the rinsing process at least 3. Figure 3, below, shows what the general titration setup should look like.
from www.chegg.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Swirl the water around a few times before opening the stopcock and allowing it to drain. For instance, you might add a standard base solution to an mystery acid solution. [2] repeat the rinsing process at least 3. As the addition takes place, the two reagents in. This is the first video in a two part. Valencia college lab technique 22: Figure 3, below, shows what the general titration setup should look like. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. The buret is held in place by the buret clamp, which is.
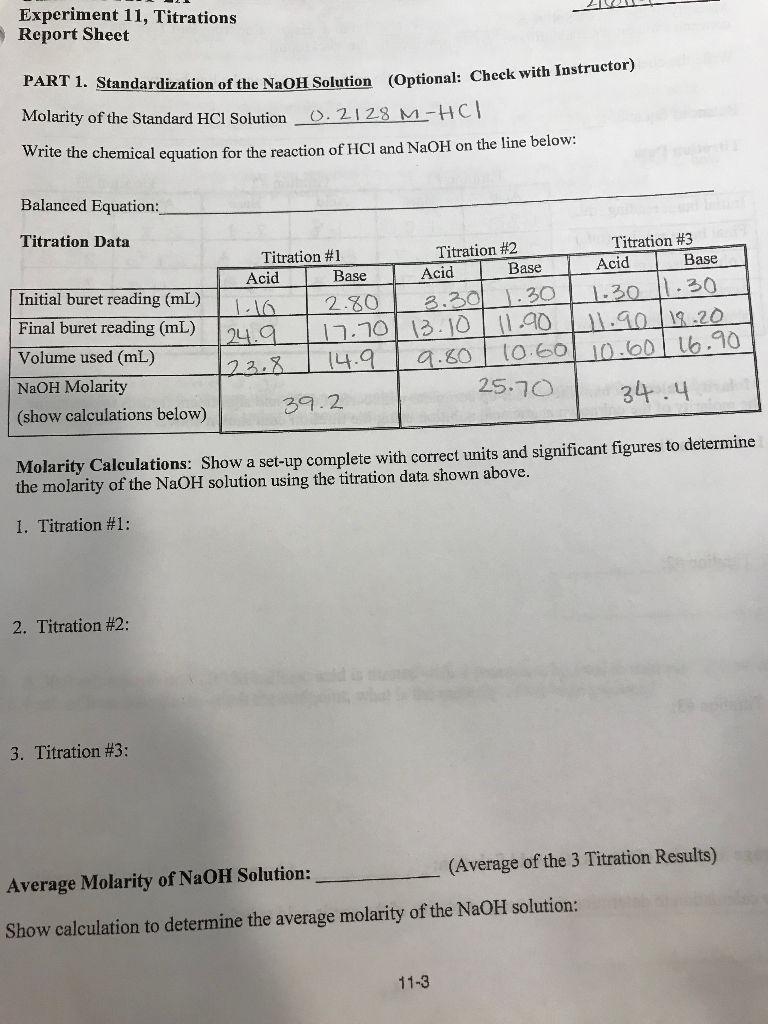
Solved Experiment 11, Titrations Report Sheet
Titration Lab Setup Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. For instance, you might add a standard base solution to an mystery acid solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. As the addition takes place, the two reagents in. Figure 3, below, shows what the general titration setup should look like. Valencia college lab technique 22: This video takes you through the proper technique for setting up and performing a titration. [2] repeat the rinsing process at least 3. Swirl the water around a few times before opening the stopcock and allowing it to drain. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The buret is held in place by the buret clamp, which is. This is the first video in a two part.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Lab Setup A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using.. Titration Lab Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Lab Setup This video takes you through the proper technique for setting up and performing a titration. As the addition takes place, the two reagents in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For instance, you might add a standard base solution to an mystery. Titration Lab Setup.
From www.colourbox.com
Titration vector illustration. Labeled Stock vector Colourbox Titration Lab Setup Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. As the addition takes place, the two reagents in. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. For instance, you might add a standard base solution. Titration Lab Setup.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Lab Setup Swirl the water around a few times before opening the stopcock and allowing it to drain. The buret is held in place by the buret clamp, which is. Figure 3, below, shows what the general titration setup should look like. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. [2] repeat. Titration Lab Setup.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab Setup This is the first video in a two part. Figure 3, below, shows what the general titration setup should look like. This video takes you through the proper technique for setting up and performing a titration. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. Titration is the slow addition of. Titration Lab Setup.
From www.youtube.com
Conductometric Titrations Lab YouTube Titration Lab Setup Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This is the first video in a two part. For instance, you might add a standard base solution. Titration Lab Setup.
From www.chegg.com
Solved Experiment 11, Titrations Report Sheet Titration Lab Setup [2] repeat the rinsing process at least 3. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow addition of one solution of a. Titration Lab Setup.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Titration Lab Setup Swirl the water around a few times before opening the stopcock and allowing it to drain. This is the first video in a two part. Valencia college lab technique 22: For instance, you might add a standard base solution to an mystery acid solution. The buret is held in place by the buret clamp, which is. This video takes you. Titration Lab Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Lab Setup As the addition takes place, the two reagents in. This video takes you through the proper technique for setting up and performing a titration. This is the first video in a two part. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is. Titration Lab Setup.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Lab Setup As the addition takes place, the two reagents in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This is the first video in a two part. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using.. Titration Lab Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Lab Setup [2] repeat the rinsing process at least 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Figure 3, below, shows what the general titration setup should look like. This is the first video in a two part. Valencia college lab technique 22: The buret. Titration Lab Setup.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Lab Setup [2] repeat the rinsing process at least 3. This is the first video in a two part. For instance, you might add a standard base solution to an mystery acid solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Valencia college lab technique 22:. Titration Lab Setup.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Titration Lab Setup This video takes you through the proper technique for setting up and performing a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Valencia college lab technique 22: As the addition takes place, the two reagents in. The buret is held in place by. Titration Lab Setup.
From hxesmboyg.blob.core.windows.net
Titration Experiment Principle at Melva Kemp blog Titration Lab Setup Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Swirl the water around a few times before opening the stopcock and allowing it to drain. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. A titration. Titration Lab Setup.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Titration Lab Setup [2] repeat the rinsing process at least 3. As the addition takes place, the two reagents in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The buret is held in place by the buret clamp, which is. For instance, you might add a standard. Titration Lab Setup.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Lab Setup This video takes you through the proper technique for setting up and performing a titration. [2] repeat the rinsing process at least 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Valencia college lab technique 22: The buret is held in place by the. Titration Lab Setup.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Lab Setup This is the first video in a two part. This video takes you through the proper technique for setting up and performing a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the. Titration Lab Setup.
From itchina.edu.mx
VEVOR Lab Stand Support Laboratory Retort Support Stand 23.6" Rod With Titration Lab Setup As the addition takes place, the two reagents in. Swirl the water around a few times before opening the stopcock and allowing it to drain. Figure 3, below, shows what the general titration setup should look like. [2] repeat the rinsing process at least 3. This is the first video in a two part. The buret is held in place. Titration Lab Setup.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Titration Lab Setup Valencia college lab technique 22: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. As the addition takes place, the two reagents in. This is the first. Titration Lab Setup.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Lab Setup As the addition takes place, the two reagents in. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Valencia college lab technique 22: Titration 4 the titration. Titration Lab Setup.
From hxetcawfs.blob.core.windows.net
Titration Apparatus Diagram at Michael Buckelew blog Titration Lab Setup Figure 3, below, shows what the general titration setup should look like. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. [2] repeat the rinsing process at. Titration Lab Setup.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Titration Lab Setup As the addition takes place, the two reagents in. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. For instance, you might add a standard base solution to an mystery acid solution. [2] repeat the rinsing process at least 3. Titration is the slow addition of one solution of a known. Titration Lab Setup.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab Setup Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. As the addition takes place, the two reagents in. For instance, you might add a standard base solution to an mystery acid solution. This is the first video in a two part. A titration is a laboratory technique used to precisely measure. Titration Lab Setup.
From www.homesciencetools.com
Titration Kit Titration Lab Equipment for Chemistry HST Titration Lab Setup The buret is held in place by the buret clamp, which is. For instance, you might add a standard base solution to an mystery acid solution. As the addition takes place, the two reagents in. Figure 3, below, shows what the general titration setup should look like. This is the first video in a two part. This video takes you. Titration Lab Setup.
From edu.rsc.org
Vintage titrations sulfur dioxide concentrations in wine Resource Titration Lab Setup A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The buret is held in place by the buret clamp, which is. Figure 3, below, shows what the. Titration Lab Setup.
From mungfali.com
Acid Base Titration Procedure Titration Lab Setup This is the first video in a two part. This video takes you through the proper technique for setting up and performing a titration. For instance, you might add a standard base solution to an mystery acid solution. [2] repeat the rinsing process at least 3. The buret is held in place by the buret clamp, which is. Titration 4. Titration Lab Setup.
From itchina.edu.mx
VEVOR Lab Stand Support Laboratory Retort Support Stand 23.6" Rod With Titration Lab Setup Figure 3, below, shows what the general titration setup should look like. [2] repeat the rinsing process at least 3. Swirl the water around a few times before opening the stopcock and allowing it to drain. Valencia college lab technique 22: This video takes you through the proper technique for setting up and performing a titration. This is the first. Titration Lab Setup.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Titration Lab Setup Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Valencia college lab technique 22: Swirl the water around a few times before opening the stopcock and allowing it to drain. Figure 3, below, shows what the general titration setup should look like. This is the. Titration Lab Setup.
From vectormine.com
Titration vector illustration VectorMine Titration Lab Setup The buret is held in place by the buret clamp, which is. Figure 3, below, shows what the general titration setup should look like. As the addition takes place, the two reagents in. [2] repeat the rinsing process at least 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Lab Setup.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Lab Setup The buret is held in place by the buret clamp, which is. For instance, you might add a standard base solution to an mystery acid solution. This video takes you through the proper technique for setting up and performing a titration. As the addition takes place, the two reagents in. Valencia college lab technique 22: Swirl the water around a. Titration Lab Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Lab Setup The buret is held in place by the buret clamp, which is. As the addition takes place, the two reagents in. [2] repeat the rinsing process at least 3. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.the basic process involves adding a standard solution of one reagent to. Titration Lab Setup.
From ar.inspiredpencil.com
Titration Diagram Titration Lab Setup This is the first video in a two part. [2] repeat the rinsing process at least 3. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. For instance, you might add a standard base solution to an mystery acid solution. Figure 3, below, shows what the general titration setup should look. Titration Lab Setup.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Lab Setup Valencia college lab technique 22: This video takes you through the proper technique for setting up and performing a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For instance, you might add a standard base solution to an mystery acid solution. This is. Titration Lab Setup.
From www.vernier.com
OxidationReduction Titrations > Experiment 19 from Investigating Titration Lab Setup This is the first video in a two part. Swirl the water around a few times before opening the stopcock and allowing it to drain. Titration 4 the titration is complete once the end point persists for at least 15 seconds (if using. Figure 3, below, shows what the general titration setup should look like. The buret is held in. Titration Lab Setup.