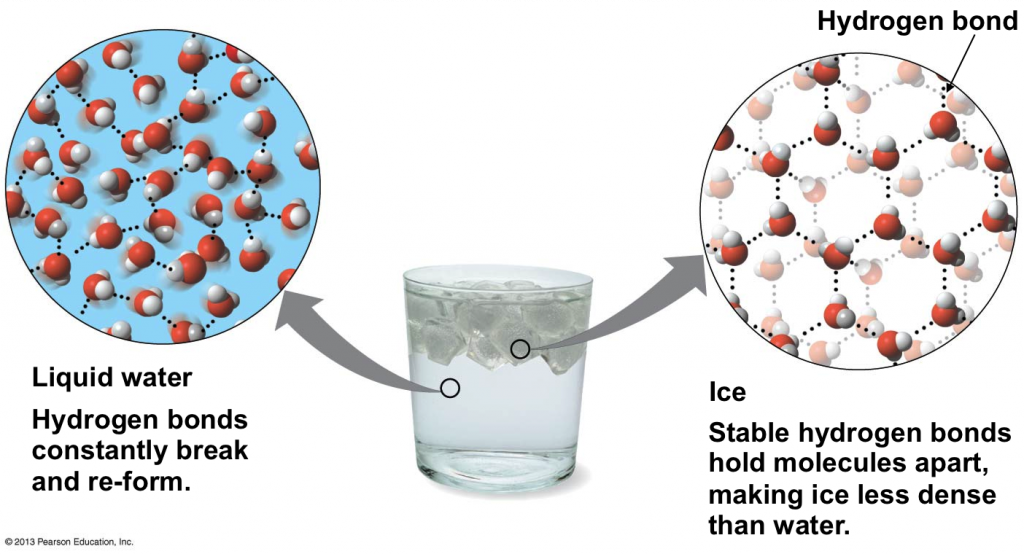
Why Does Salt Not Freeze . For example, calcium chloride lowers the freezing point more than sodium chloride. Salt makes ice colder because the salt prevents melted water from freezing. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Melting is endothermic, so it lowers the temperature. As you add more salt to water, the freezing point drops. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. As salt is added to this water, its freezing points starts. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. The more salt you add, the lower the freezing point becomes. Salt helps melt ice and prevent it. That’s why many cities spray a salt solution before any ice forms. This phenomenon is called freezing point depression. This lowers the freezing point of ocean water. In addition to melting ice, freezing point depression can. In water, salt is a solute, and it will break into its elements.
from punchlistzero.com
This phenomenon is called freezing point depression. As salt is added to this water, its freezing points starts. Salt makes ice colder because the salt prevents melted water from freezing. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. In addition to melting ice, freezing point depression can. As you add more salt to water, the freezing point drops. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. The more salt you add, the lower the freezing point becomes. This concept is called “freezing point depression.”. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water.
Specific Heat of Ice In Various Units, vs. Water,
Why Does Salt Not Freeze In addition to melting ice, freezing point depression can. This concept is called “freezing point depression.”. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. In addition to melting ice, freezing point depression can. For example, calcium chloride lowers the freezing point more than sodium chloride. That’s why many cities spray a salt solution before any ice forms. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). This phenomenon is called freezing point depression. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. The more salt you add, the lower the freezing point becomes. This lowers the freezing point of ocean water. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. In water, salt is a solute, and it will break into its elements.
From www.newscientist.com
Why does my water freeze in this pattern like dandelion fluff? New Why Does Salt Not Freeze As salt is added to this water, its freezing points starts. For example, calcium chloride lowers the freezing point more than sodium chloride. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. The working temperature range isn't the same for all types of salt. Melting is endothermic, so it lowers the temperature.. Why Does Salt Not Freeze.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Does Salt Not Freeze This lowers the freezing point of ocean water. Melting is endothermic, so it lowers the temperature. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). As you add more salt to water, the freezing point drops. In water, salt is a solute, and it will break into. Why Does Salt Not Freeze.
From www.animalia-life.club
Freezing Of Water Why Does Salt Not Freeze In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. This. Why Does Salt Not Freeze.
From pagingfunmums.com
Melting Ice & Salt Science Experiment Why Does Salt Not Freeze The working temperature range isn't the same for all types of salt. Melting is endothermic, so it lowers the temperature. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). Salt makes ice colder because the salt prevents melted water from freezing. That’s why many cities spray a. Why Does Salt Not Freeze.
From www.reddit.com
If solids are denser than liquids, why do liquids expand when they Why Does Salt Not Freeze Salt helps melt ice and prevent it. This lowers the freezing point of ocean water. This concept is called “freezing point depression.”. That’s why many cities spray a salt solution before any ice forms. In addition to melting ice, freezing point depression can. For example, calcium chloride lowers the freezing point more than sodium chloride. Salt makes ice colder because. Why Does Salt Not Freeze.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. Salt helps melt ice and prevent it. In addition to melting ice, freezing point depression can. As salt is added to this water, its freezing points starts. For example, calcium chloride lowers the freezing point more than sodium chloride. Essentially, the salt makes it harder for the. Why Does Salt Not Freeze.
From howtofixit.net
Why Is My Refrigerator Working but Freezer Not Freezing? How To Fix It Why Does Salt Not Freeze The working temperature range isn't the same for all types of salt. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Salt makes ice colder because the salt prevents melted water from freezing. That’s why many cities spray a salt solution before any ice forms. Essentially, the salt makes. Why Does Salt Not Freeze.
From selectsalt.com
The Nutritional Powerhouse of FreezeDried Fruits Select Salt Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. This phenomenon is called freezing point depression. For example, calcium chloride lowers the freezing point more than sodium chloride. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Although the saltiness of ocean water varies, often. Why Does Salt Not Freeze.
From www.worldatlas.com
What Are The 10 Physical Changes? WorldAtlas Why Does Salt Not Freeze That’s why many cities spray a salt solution before any ice forms. Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. This lowers the freezing point of ocean water. Salt helps melt ice and prevent it. As you add more salt to water, the freezing point drops. So, if you’re using table salt, also. Why Does Salt Not Freeze.
From www.youtube.com
Supercooled Water Instant Freeze & Snowflakes in a Bottle YouTube Why Does Salt Not Freeze This phenomenon is called freezing point depression. Salt helps melt ice and prevent it. The working temperature range isn't the same for all types of salt. In addition to melting ice, freezing point depression can. Melting is endothermic, so it lowers the temperature. In water, salt is a solute, and it will break into its elements. Salt that’s dumped on. Why Does Salt Not Freeze.
From exovisoqz.blob.core.windows.net
Does Salt Dissolve In Salt Water at Tomas Hartt blog Why Does Salt Not Freeze The more salt you add, the lower the freezing point becomes. That’s why many cities spray a salt solution before any ice forms. As salt is added to this water, its freezing points starts. In addition to melting ice, freezing point depression can. The working temperature range isn't the same for all types of salt. For example, calcium chloride lowers. Why Does Salt Not Freeze.
From www.youtube.com
How to Turn Water into Ice in Seconds Explanation Tutorial YouTube Why Does Salt Not Freeze This phenomenon is called freezing point depression. Salt makes ice colder because the salt prevents melted water from freezing. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. In water, salt is a solute, and it will break into its elements. As salt is. Why Does Salt Not Freeze.
From www.youtube.com
Salt, Sugar or Hot Water Which melts ice faster? YouTube Why Does Salt Not Freeze For example, calcium chloride lowers the freezing point more than sodium chloride. Salt makes ice colder because the salt prevents melted water from freezing. Melting is endothermic, so it lowers the temperature. That’s why many cities spray a salt solution before any ice forms. This lowers the freezing point of ocean water. In case of sea water, the average salt. Why Does Salt Not Freeze.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Why Does Salt Not Freeze Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. As you add more salt to water, the freezing point drops. Salt makes ice colder because the salt prevents melted water from freezing. This concept is called “freezing point depression.”. In water, salt is a solute, and it will break into its elements.. Why Does Salt Not Freeze.
From selectsalt.com
Winter Road Salt Select Salt Why Does Salt Not Freeze That’s why many cities spray a salt solution before any ice forms. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. This lowers the freezing point of ocean water. Salt makes ice colder because the salt prevents melted water from freezing. As salt is added to this water, its freezing points starts.. Why Does Salt Not Freeze.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Why Does Salt Not Freeze As you add more salt to water, the freezing point drops. This phenomenon is called freezing point depression. As salt is added to this water, its freezing points starts. Melting is endothermic, so it lowers the temperature. This concept is called “freezing point depression.”. For example, calcium chloride lowers the freezing point more than sodium chloride. Although the saltiness of. Why Does Salt Not Freeze.
From www.nutriinspector.com
Can Salt Water Freeze Exploring the Salt's Frozen Depths Nutri Inspector Why Does Salt Not Freeze In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. As you add more salt to water, the freezing point drops. The working. Why Does Salt Not Freeze.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Why Does Salt Not Freeze As salt is added to this water, its freezing points starts. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). The more salt you add, the lower the freezing point becomes. Melting is endothermic, so it lowers the temperature. Essentially, the salt makes it harder for the. Why Does Salt Not Freeze.
From jacksofscience.com
What Temp Does Salt Water Freeze? Jacks Of Science Why Does Salt Not Freeze For example, calcium chloride lowers the freezing point more than sodium chloride. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). This lowers the freezing point of. Why Does Salt Not Freeze.
From sciencenotes.org
Freezing Point Depression Formula and Definition Why Does Salt Not Freeze This phenomenon is called freezing point depression. This lowers the freezing point of ocean water. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. Salt makes ice colder because the salt prevents melted water from freezing. The working temperature range isn't the same for all types of. Why Does Salt Not Freeze.
From techiescientist.com
Why Does Salt Make Ice Colder? Techiescientist Why Does Salt Not Freeze Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. Salt makes ice colder because the salt prevents melted water from freezing. This concept is called “freezing point. Why Does Salt Not Freeze.
From www.morningagclips.com
Why does salt change the taste of everything? Morning Ag Clips Why Does Salt Not Freeze As salt is added to this water, its freezing points starts. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. This lowers the freezing point of ocean water. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c. Why Does Salt Not Freeze.
From www.flickr.com
Does Salt Water Freeze Pictures for Emily's Science Fair p… Flickr Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. That’s why many cities spray a salt solution before any ice forms. Salt makes ice colder because the salt prevents melted water from freezing. As salt is added to this water, its freezing points starts. Although the saltiness of ocean water varies, often ocean water has about. Why Does Salt Not Freeze.
From www.pinterest.com
Saltwater Experiment freeze water at different temperatures to see Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. The more salt you add, the lower the freezing point becomes. Melting is endothermic, so it lowers the temperature. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. This phenomenon is. Why Does Salt Not Freeze.
From lenardfdleckman.blob.core.windows.net
Does Ice Melt Faster On Metal Or Wood at lenardfdleckman blog Why Does Salt Not Freeze In addition to melting ice, freezing point depression can. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. Melting is endothermic, so it lowers the temperature. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Essentially,. Why Does Salt Not Freeze.
From farmfoodfamily.com
10 Reasons Why LG Freezer Not Freezing/Working {How to Fix} Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. The more salt you add, the lower the freezing point becomes. This concept is called “freezing point depression.”. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Essentially, the salt makes it harder for the water. Why Does Salt Not Freeze.
From www.artofit.org
Infographic the difference between freeze and shutdown trauma responses Why Does Salt Not Freeze That’s why many cities spray a salt solution before any ice forms. This phenomenon is called freezing point depression. The more salt you add, the lower the freezing point becomes. In addition to melting ice, freezing point depression can. Salt makes ice colder because the salt prevents melted water from freezing. This concept is called “freezing point depression.”. Salt helps. Why Does Salt Not Freeze.
From beezzly.com
Why Do Some Water Bottles Freeze And Others Don't? Beezzly Why Does Salt Not Freeze This concept is called “freezing point depression.”. Essentially, the salt makes it harder for the water molecules to bond together in their rigid structure. Melting is endothermic, so it lowers the temperature. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. In addition to melting ice, freezing. Why Does Salt Not Freeze.
From www.worksheetsplanet.com
What is Freezing Definition of Freezing Why Does Salt Not Freeze The more salt you add, the lower the freezing point becomes. As salt is added to this water, its freezing points starts. In case of sea water, the average salt content is 35 parts per thousand, and therefore, its freezing point is −1.8°c (−28.9°f). Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. Salt. Why Does Salt Not Freeze.
From epizouu8wmaterialdb.z21.web.core.windows.net
Distillation Of Salt Water Why Does Salt Not Freeze As you add more salt to water, the freezing point drops. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Salt helps melt ice and prevent it. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially. Why Does Salt Not Freeze.
From dkijlydeeco.blob.core.windows.net
Why Does Salt And Ice Freeze Water at Michelle Fisher blog Why Does Salt Not Freeze In water, salt is a solute, and it will break into its elements. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. Salt that’s dumped on top of ice relies on the sun or the friction of car tires driving over it to initially melt the ice. The more. Why Does Salt Not Freeze.
From studylib.net
Freezing Point of Salt Water .docx Why Does Salt Not Freeze Salt helps melt ice and prevent it. Melting is endothermic, so it lowers the temperature. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. That’s why many cities spray a salt solution before any ice forms. This concept is called “freezing point depression.”. Salt makes ice colder. Why Does Salt Not Freeze.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube Why Does Salt Not Freeze In addition to melting ice, freezing point depression can. This phenomenon is called freezing point depression. For example, calcium chloride lowers the freezing point more than sodium chloride. Melting is endothermic, so it lowers the temperature. This concept is called “freezing point depression.”. Salt makes ice colder because the salt prevents melted water from freezing. The more salt you add,. Why Does Salt Not Freeze.
From www.researchgate.net
Freezing temperatures of salt brine mixtures Download Scientific Diagram Why Does Salt Not Freeze This concept is called “freezing point depression.”. Melting is endothermic, so it lowers the temperature. As you add more salt to water, the freezing point drops. Although the saltiness of ocean water varies, often ocean water has about 35 grams of salt for every 1,000 units of water. Salt helps melt ice and prevent it. Salt that’s dumped on top. Why Does Salt Not Freeze.
From www.youtube.com
Why Does Salt Make Food Taste Better? YouTube Why Does Salt Not Freeze In addition to melting ice, freezing point depression can. This phenomenon is called freezing point depression. The more salt you add, the lower the freezing point becomes. For example, calcium chloride lowers the freezing point more than sodium chloride. So, if you’re using table salt, also known as sodium chloride (nacl), to melt ice, the salt will dissolve into. This. Why Does Salt Not Freeze.