Energy Calories Heat . the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. Historically, energy was measured in units of calories (cal). the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. This conversion factor between units will not be used in these notes, and all quantities expressing heat. describe the meaning and origins of chemical energy. measuring energy and heat capacity. The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. A calorie is the amount of. calorie, a unit of energy or heat variously defined.
from classnotes.org.in
This conversion factor between units will not be used in these notes, and all quantities expressing heat. measuring energy and heat capacity. The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. Historically, energy was measured in units of calories (cal). A calorie is the amount of. describe the meaning and origins of chemical energy. the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. calorie, a unit of energy or heat variously defined.
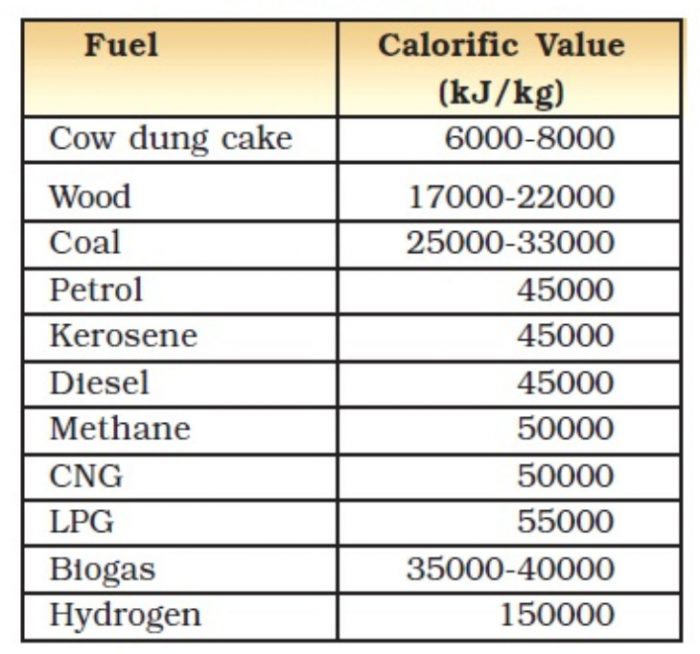
Fuels Class 10, Sources of Energy
Energy Calories Heat A calorie is the amount of. describe the meaning and origins of chemical energy. the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. calorie, a unit of energy or heat variously defined. Historically, energy was measured in units of calories (cal). A calorie is the amount of. This conversion factor between units will not be used in these notes, and all quantities expressing heat. measuring energy and heat capacity.
From www.slideshare.net
Heat Energy Energy Calories Heat describe the meaning and origins of chemical energy. This conversion factor between units will not be used in these notes, and all quantities expressing heat. Historically, energy was measured in units of calories (cal). another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00. Energy Calories Heat.
From www.slideserve.com
PPT CALORIES PowerPoint Presentation, free download ID2820337 Energy Calories Heat Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. A calorie is the amount of. calorie, a unit of energy or heat variously. Energy Calories Heat.
From caloriebee.com
Weight Watchers Points Calculation Tips CalorieBee Energy Calories Heat A calorie is the amount of. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. Historically, energy was measured in units of calories (cal).. Energy Calories Heat.
From haipernews.com
How To Calculate Joules Of Heat Energy Haiper Energy Calories Heat The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. This conversion factor between units will not be used in these notes, and all quantities expressing heat. the conversion factor between the two units, j, is the mechanical equivalent of heat,. Energy Calories Heat.
From imathworks.com
[Physics] Thermodynamics Units in calculations of heat (Q) Math Energy Calories Heat A calorie is the amount of. calorie, a unit of energy or heat variously defined. describe the meaning and origins of chemical energy. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. measuring energy and heat capacity. the conversion. Energy Calories Heat.
From laughingsquid.com
An Animated Explanation of Calories and How Many Are Really Needed by Energy Calories Heat calorie, a unit of energy or heat variously defined. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. measuring energy and heat capacity. This conversion factor between units will not be used. Energy Calories Heat.
From www.slideserve.com
PPT Heat and Temperature Change PowerPoint Presentation, free Energy Calories Heat Historically, energy was measured in units of calories (cal). another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. Heat and work are both expressed in energy units, but they differ from plain energy in. Energy Calories Heat.
From www.slideserve.com
PPT Chapter 11 Energy in Thermal Process PowerPoint Presentation Energy Calories Heat another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. A calorie is the amount of. measuring energy and heat capacity. This conversion factor between units will not be used in these notes, and. Energy Calories Heat.
From www.slideserve.com
PPT Heat & Thermodynamics PowerPoint Presentation, free download ID Energy Calories Heat the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. Historically, energy was measured in units of calories (cal). The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°.. Energy Calories Heat.
From studylib.net
1. A calorie is a unit of energy OG Energy Calories Heat the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. This conversion factor between units will not be used in these notes, and all quantities expressing heat. another common unit of energy often used for heat is the calorie (cal), defined as the. Energy Calories Heat.
From www.youtube.com
Lesson Video Calories and Energy YouTube Energy Calories Heat calorie, a unit of energy or heat variously defined. describe the meaning and origins of chemical energy. the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. This conversion factor between units will not be used in these notes, and all quantities expressing heat. A. Energy Calories Heat.
From www.slideserve.com
PPT EPE Calories Law of Conservation of Energy and WorkEnergy Energy Calories Heat A calorie is the amount of. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way.. Energy Calories Heat.
From www.slideserve.com
PPT Heat and Thermal Energy PowerPoint Presentation, free download Energy Calories Heat Historically, energy was measured in units of calories (cal). another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. This conversion factor between units will not be used in these notes, and all quantities expressing. Energy Calories Heat.
From www.showme.com
Heat capacity turning to calories Science, Chemistry ShowMe Energy Calories Heat the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. measuring energy and heat capacity. A calorie is the amount of. describe the meaning and origins of chemical energy. The calorie was originally defined as the amount of heat required at a. Energy Calories Heat.
From healthappsuk.blogspot.com
Health Apps What is a calorie and why are they so important? Energy Calories Heat calorie, a unit of energy or heat variously defined. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. describe the meaning and origins of chemical energy. Historically, energy was measured in units of calories (cal). A calorie is the amount of.. Energy Calories Heat.
From slidetodoc.com
Temperature Heat Thermal Expansion and Heat Transfer PHYSICS Energy Calories Heat Historically, energy was measured in units of calories (cal). This conversion factor between units will not be used in these notes, and all quantities expressing heat. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. The calorie was originally defined as the amount. Energy Calories Heat.
From www.heandsheeatclean.com
Eating Clean Means Consuming Calories! Energy Calories Heat another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. This conversion factor between units will not be used in these notes, and all quantities expressing heat. The calorie was originally defined as the amount. Energy Calories Heat.
From www.scribd.com
Thermal Energy and Heat PDF Calorie Heat Energy Calories Heat describe the meaning and origins of chemical energy. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. calorie, a unit of energy or heat variously defined. measuring energy and heat capacity.. Energy Calories Heat.
From www.slideserve.com
PPT Energy in Earth Processes PowerPoint Presentation, free download Energy Calories Heat Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. describe the meaning and origins of chemical energy. the heat capacity. Energy Calories Heat.
From gamesmartz.com
Calorie Definition Easy to Understand Energy Calories Heat measuring energy and heat capacity. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. This conversion factor between units will not be used in these notes, and all quantities expressing heat. Historically, energy was measured in units of calories (cal). A calorie is the amount of. another common. Energy Calories Heat.
From classnotes.org.in
Fuels Class 10, Sources of Energy Energy Calories Heat Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. Historically, energy was measured in units of calories (cal). This conversion factor between units will not be used in these notes, and all quantities expressing heat. the conversion factor between the two units, j, is the mechanical equivalent of heat,. Energy Calories Heat.
From fitnesssoup.com
Eating to Lose Weight What Can I Eat and Lose Weight? Energy Calories Heat measuring energy and heat capacity. calorie, a unit of energy or heat variously defined. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. A calorie is the amount of. This conversion factor between units will not be used in these notes,. Energy Calories Heat.
From www.inchcalculator.com
Calories Burned Calculator Inch Calculator Energy Calories Heat measuring energy and heat capacity. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. calorie, a unit of. Energy Calories Heat.
From www.chegg.com
Solved Match the gain or loss of heat energy with the Energy Calories Heat This conversion factor between units will not be used in these notes, and all quantities expressing heat. A calorie is the amount of. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. measuring energy and heat capacity. calorie, a unit of energy or heat variously defined. another. Energy Calories Heat.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation, free download ID Energy Calories Heat the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. A calorie is the amount of. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. the conversion factor between the two units, j,. Energy Calories Heat.
From haipernews.com
How To Calculate The Heat Capacity Haiper Energy Calories Heat Historically, energy was measured in units of calories (cal). measuring energy and heat capacity. This conversion factor between units will not be used in these notes, and all quantities expressing heat. describe the meaning and origins of chemical energy. A calorie is the amount of. the conversion factor between the two units, j, is the mechanical equivalent. Energy Calories Heat.
From www.youtube.com
Heat & Energy Unit Conversions YouTube Energy Calories Heat Historically, energy was measured in units of calories (cal). Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. calorie, a unit of energy or heat variously defined. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases. Energy Calories Heat.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID6317093 Energy Calories Heat the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. Historically, energy was measured in units of calories (cal). describe. Energy Calories Heat.
From logherscience10.blogspot.com
Science 10 Energy Transfers all around us Energy Calories Heat the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. This conversion factor between units will not be used in these notes, and all quantities expressing heat. Historically, energy was measured in units of calories (cal). the heat capacity ( c) of a body of matter. Energy Calories Heat.
From www.digitalbarbell.com
energy — Practical Training, Nutrition and Fitness Advice You Can Trust Energy Calories Heat the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. This conversion factor between units will not be used in these notes, and all quantities expressing heat. calorie, a unit of energy or heat variously defined. A calorie is the amount of. Historically, energy was measured. Energy Calories Heat.
From fitnessprogramer.com
Understanding Energy And Calories / Workout Builder Energy Calories Heat the conversion factor between the two units, j, is the mechanical equivalent of heat, or the number of joules in a calorie. measuring energy and heat capacity. A calorie is the amount of. This conversion factor between units will not be used in these notes, and all quantities expressing heat. another common unit of energy often used. Energy Calories Heat.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID6836296 Energy Calories Heat describe the meaning and origins of chemical energy. another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oc\)—specifically, between \(14.5^oc\) and \(15.5^oc\) since. Heat and work are both expressed in energy units, but they differ from plain energy in. Energy Calories Heat.
From in.pinterest.com
What are calories? Follow ketodietactive Calories are a measure of Energy Calories Heat The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. measuring energy and heat capacity. This conversion factor between units will not be used in these notes, and all quantities expressing heat. the conversion factor between the two units, j,. Energy Calories Heat.
From www.slideserve.com
PPT Units of Energy PowerPoint Presentation, free download ID5685581 Energy Calories Heat describe the meaning and origins of chemical energy. The calorie was originally defined as the amount of heat required at a pressure of 1 standard atmosphere to raise the temperature of 1 gram of water 1°. calorie, a unit of energy or heat variously defined. the conversion factor between the two units, j, is the mechanical equivalent. Energy Calories Heat.
From studylib.net
Heat Equation Energy Calories Heat the heat capacity ( c) of a body of matter is the quantity of heat ( q) it absorbs or releases when it experiences a. Heat and work are both expressed in energy units, but they differ from plain energy in a fundamental way. measuring energy and heat capacity. the conversion factor between the two units, j,. Energy Calories Heat.