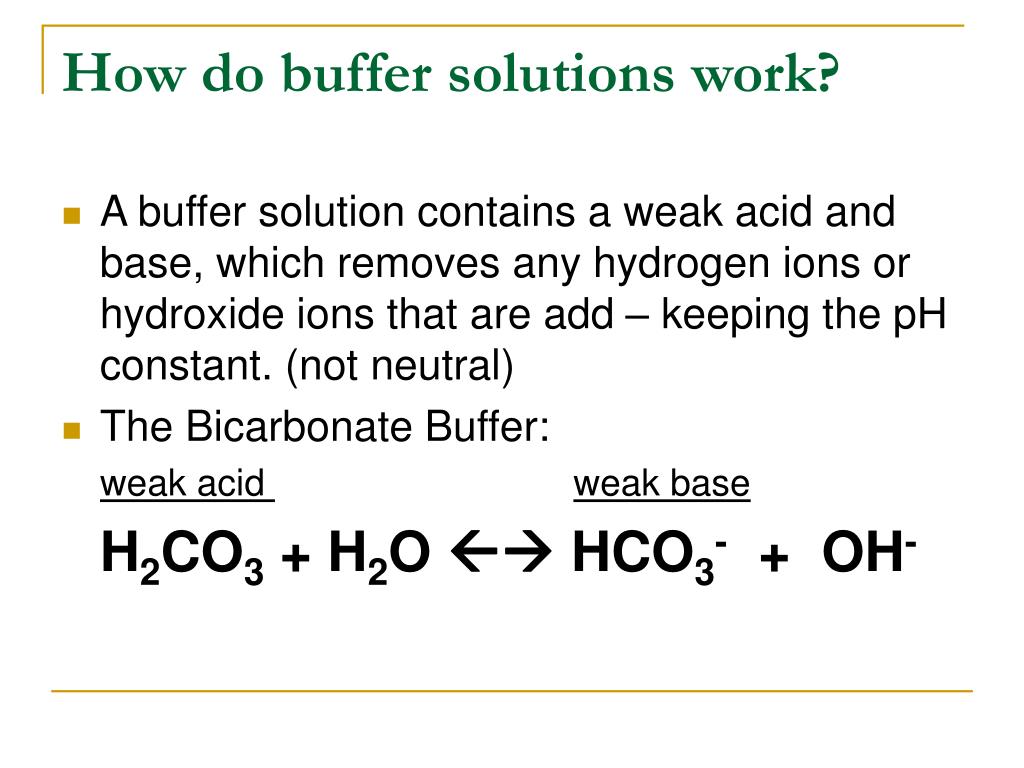
Explain How Buffers Work . To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. What is a buffer composed of? Selecting proper components for desired ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. Buffers do so by being composed of certain pairs of solutes: Each buffer is characterized by a set. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. This tutorial describes how buffers protect against ph changes when strong acid or base is added. Buffer solutions resist a change in ph. Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. How does a buffer work?
from www.slideserve.com
To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. How does a buffer work? Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers do so by being composed of certain pairs of solutes: This tutorial describes how buffers protect against ph changes when strong acid or base is added. Each buffer is characterized by a set. Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph.
PPT Buffers PowerPoint Presentation, free download ID5687114
Explain How Buffers Work To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. What is a buffer composed of? Selecting proper components for desired ph. Buffer solutions resist a change in ph. This tutorial describes how buffers protect against ph changes when strong acid or base is added. Each buffer is characterized by a set. Buffers do so by being composed of certain pairs of solutes: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. How does a buffer work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Explain How Buffers Work This tutorial describes how buffers protect against ph changes when strong acid or base is added. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. How does a buffer work? Each buffer is characterized by a set. To illustrate the. Explain How Buffers Work.
From www.numerade.com
SOLVED Please type the answer Explain the importance of the protein Explain How Buffers Work Each buffer is characterized by a set. Selecting proper components for desired ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. What is a buffer composed of? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of. Explain How Buffers Work.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Explain How Buffers Work Each buffer is characterized by a set. Either a weak acid plus a salt derived from that. Buffers do so by being composed of certain pairs of solutes: Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer. Explain How Buffers Work.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Explain How Buffers Work Each buffer is characterized by a set. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that. How does a buffer work? What is a buffer composed of? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Explain How Buffers Work.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Explain How Buffers Work How does a buffer work? Either a weak acid plus a salt derived from that. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffers do so by being. Explain How Buffers Work.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download Explain How Buffers Work Each buffer is characterized by a set. Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? Either a weak acid plus a salt derived from that. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Explain How Buffers Work.
From saylordotorg.github.io
Buffers Explain How Buffers Work A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. Either a weak acid plus a salt derived from that. Buffer solutions resist a change in ph. Each buffer is characterized by a set. The mechanism involves a buffer, a solution that resists dramatic changes. Explain How Buffers Work.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Explain How Buffers Work Buffer solutions resist a change in ph. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Each buffer is characterized by a set. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffers do so by being composed. Explain How Buffers Work.
From www.brainkart.com
How do buffers work? Explain How Buffers Work Selecting proper components for desired ph. This tutorial describes how buffers protect against ph changes when strong acid or base is added. Buffers do so by being composed of certain pairs of solutes: Buffer solutions resist a change in ph. How does a buffer work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts. Explain How Buffers Work.
From courses.lumenlearning.com
Buffers Chemistry Explain How Buffers Work Buffer solutions resist a change in ph. This tutorial describes how buffers protect against ph changes when strong acid or base is added. How does a buffer work? Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that resists dramatic changes in ph. To illustrate the function of a buffer solution,. Explain How Buffers Work.
From www.vrogue.co
How Buffers Work vrogue.co Explain How Buffers Work Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that. How does a buffer work? The mechanism involves a buffer, a solution that resists dramatic changes in ph. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Selecting. Explain How Buffers Work.
From www.osmosis.org
Making buffer solutions Osmosis Explain How Buffers Work Selecting proper components for desired ph. Buffers do so by being composed of certain pairs of solutes: A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. Either a weak acid plus a salt derived from that. How does a buffer work? Each buffer is. Explain How Buffers Work.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Explain How Buffers Work Buffers do so by being composed of certain pairs of solutes: This tutorial describes how buffers protect against ph changes when strong acid or base is added. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. To illustrate the function. Explain How Buffers Work.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Explain How Buffers Work Selecting proper components for desired ph. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. To illustrate the function of a buffer solution, consider a mixture of. Explain How Buffers Work.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Explain How Buffers Work Either a weak acid plus a salt derived from that. Buffers do so by being composed of certain pairs of solutes: To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a. Explain How Buffers Work.
From www.slideserve.com
PPT Introduction to Buffers PowerPoint Presentation, free download Explain How Buffers Work Buffer solutions resist a change in ph. What is a buffer composed of? A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from. Explain How Buffers Work.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 Explain How Buffers Work Selecting proper components for desired ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. What is a buffer composed of? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Explain How Buffers Work.
From www.slideserve.com
PPT 18 Additional Aspects of AcidBase Equilibria PowerPoint Explain How Buffers Work Buffer solutions resist a change in ph. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. What is a buffer composed of? Selecting proper components for desired ph. Each buffer is characterized by a set. Buffers do so by being composed of certain pairs of solutes: The mechanism involves. Explain How Buffers Work.
From www.pinterest.com.au
Buffer solutions Buffer solution, Chemistry lessons, Ap chemistry Explain How Buffers Work What is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that. How does a buffer work? A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. Selecting proper components. Explain How Buffers Work.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Explain How Buffers Work The mechanism involves a buffer, a solution that resists dramatic changes in ph. Each buffer is characterized by a set. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Either a weak acid plus a salt derived from that. How does a buffer work? What is a buffer composed. Explain How Buffers Work.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Explain How Buffers Work To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. A mixture of a weak acid and its conjugate base (or a mixture of a. Explain How Buffers Work.
From www.youtube.com
How Do Buffers Work? With Buffer Calculations! AP Chemistry Unit 8.3 Explain How Buffers Work To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Each buffer is characterized by a set. The mechanism involves a buffer, a solution that resists dramatic changes in ph. This tutorial describes how buffers protect against ph changes when strong acid or base is added. Buffer solutions resist a. Explain How Buffers Work.
From www.slideserve.com
PPT Chapter 17 Applications of Aqueous Equilibria PowerPoint Explain How Buffers Work Either a weak acid plus a salt derived from that. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition. Explain How Buffers Work.
From www.slideshare.net
Ch 18 buffers Explain How Buffers Work The mechanism involves a buffer, a solution that resists dramatic changes in ph. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Each buffer is characterized by a set.. Explain How Buffers Work.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 Explain How Buffers Work Buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired ph. How does a buffer work? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. To illustrate the function of a buffer solution, consider. Explain How Buffers Work.
From www.slideserve.com
PPT Today’s Objective SWBAT explain how a buffer works. SWBAT Explain How Buffers Work To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. This tutorial describes how buffers protect against ph changes when strong acid or base is added. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A mixture of a weak acid and its conjugate base (or. Explain How Buffers Work.
From www.slideshare.net
2012 topic 18 2 buffer solutions Explain How Buffers Work Either a weak acid plus a salt derived from that. How does a buffer work? Each buffer is characterized by a set. This tutorial describes how buffers protect against ph changes when strong acid or base is added. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. What is. Explain How Buffers Work.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists Explain How Buffers Work A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. How does a buffer work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffers do so by being composed. Explain How Buffers Work.
From www.youtube.com
More About Buffers (A2 Chemistry) YouTube Explain How Buffers Work What is a buffer composed of? Either a weak acid plus a salt derived from that. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. How does a buffer work? Each buffer is characterized by a set. Selecting proper components for desired ph. To illustrate the function of a. Explain How Buffers Work.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Explain How Buffers Work Either a weak acid plus a salt derived from that. What is a buffer composed of? Buffer solutions resist a change in ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. How does a buffer work? Each buffer is characterized by a set.. Explain How Buffers Work.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Explain How Buffers Work What is a buffer composed of? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that.. Explain How Buffers Work.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Explain How Buffers Work A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Either a weak acid plus a salt derived from that. Selecting proper components for desired. Explain How Buffers Work.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Explain How Buffers Work A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Each buffer is characterized by a set. Either a weak acid plus a salt derived from that. How does a buffer work? To illustrate the function of a buffer solution, consider. Explain How Buffers Work.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Explain How Buffers Work This tutorial describes how buffers protect against ph changes when strong acid or base is added. Selecting proper components for desired ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers do so by being composed of certain pairs. Explain How Buffers Work.
From www.slideshare.net
Buffer system Explain How Buffers Work A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Either a weak acid plus a salt derived from that. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount. Explain How Buffers Work.